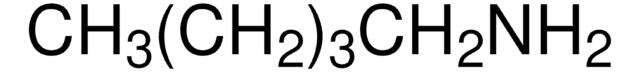171409
Amylamine
99%
Synonym(s):
Pentylamine, 1-Aminopentane, n-Amylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4NH2
CAS Number:
Molecular Weight:
87.16
Beilstein:
505953
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.01 (vs air)
Quality Level
Assay
99%
form
liquid
expl. lim.
22 %
refractive index
n20/D 1.411 (lit.)
bp
104 °C (lit.)
mp
−50 °C (lit.)
density
0.752 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCCCCN
InChI
1S/C5H13N/c1-2-3-4-5-6/h2-6H2,1H3
InChI key
DPBLXKKOBLCELK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Amylamine can be used in the imidization of 2,6-dibromonaphthalene and 2,3,6,7-tetrabromonaphthalene bisanhydride, which are used to synthesize polybromo naphthalenetetracarboxylic acid diimides (NDIs).
It can be also be used to synthesize:
It can be also be used to synthesize:
- N
- -pentyl side chains of a cyclic heptapeptoid which forms the core of verticilide
- N-pentyl sulfamides from sulfamate salts.
Amylamine is a general reagent used in functionalizing the target molecules with pentyl chain. It has also been used as a cosurfactant to increase the phase stability of the bilayer systems.
Caution
May darken in storage.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
44.6 °F
Flash Point(C)
7 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Substituted heterocyclic naphthalene diimides with unexpected acidity. Synthesis, properties, and reactivity
Doria F, et al.
The Journal of Organic Chemistry, 74(22), 8616-8625 (2009)
Efficient Synthesis of 5H-Cyclopenta [c] quinoline Derivatives via Palladium-Catalyzed Domino Reactions of o-Alkynylhalobenzene with Amine.
Luo Y, et al.
Organic Letters, 13(5), 1150-1153 (2011)
Microwave-mediated synthesis of a cyclic heptapeptoid through consecutive Ugi reactions
Barreto AS and Andrade CZ
Tetrahedron, 74(48), 6861-6865 (2018)
Investigations on the 4-quinolone-3-carboxylic acid motif. 7. Synthesis and pharmacological evaluation of 4-quinolone-3-carboxamides and 4-hydroxy-2-quinolone-3-carboxamides as high affinity cannabinoid receptor 2 (CB2R) ligands with improved aqueous solubility.
Mugnaini C, et al.
Journal of Medicinal Chemistry, 59(3), 1052-1067 (2016)
Synthesis of nanosized silver platelets in octylamine-water bilayer systems.
Yener D O, et al.
Langmuir, 18(22), 8692-8699 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service