204714
Tin(IV) oxide
≥99.99% trace metals basis
Synonym(s):
Stannic oxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
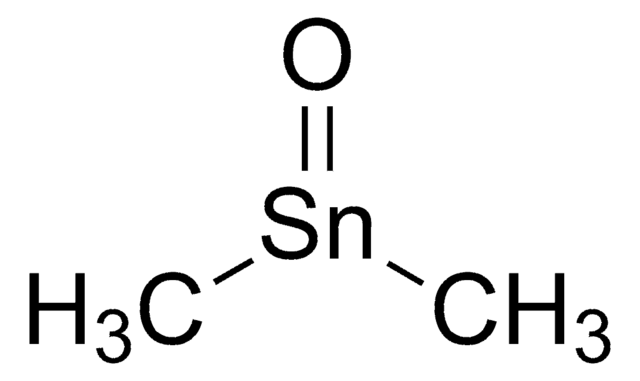
SnO2
CAS Number:
Molecular Weight:
150.71
EC Number:
MDL number:
UNSPSC Code:
12352303
eCl@ss:
38140208
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
powder and chunks
density
6.95 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Sn]=O
InChI
1S/2O.Sn
InChI key
XOLBLPGZBRYERU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tin(IV) oxide, also known as stannic oxide, is a yellow-green powder that crystallizes in the rutile structure. It is a wide bandgap (3.6 eV) semiconductor with high transparency in the visible range of the electromagnetic spectrum and relatively high electronic conductivity. Its chemical stability and high purity of ≥99.99% trace metals basis make it suitable for use in demanding conditions, such as semiconductor and biomedical applications, where it is widely used in medical imaging devices, biosensors, and diagnostic tools. It is also utilized in battery technologies, including lithium-ion batteries, as a conversion-type anode material due to its high energy storage capacity and stability and a precursor for making tin compounds and complex metal oxides.
Application
- Fluorinated Cation-Based 2D Perovskites for Efficient and Stable 3D/2D Heterojunction Perovskite Solar Cells.: This research explores the application of tin(IV) oxide in creating efficient and stable perovskite solar cells, focusing on the improvement of the solar cells′ overall performance (Shaw PE et al., 2023).
- Tin(IV) Oxide Electron Transport Layer via Industrial-Scale Pulsed Laser Deposition for Planar Perovskite Solar Cells.: The study discusses the use of tin(IV) oxide as an electron transport layer applied through industrial-scale pulsed laser deposition, enhancing the functionality and efficiency of planar perovskite solar cells (Bolink HJ et al., 2023).
- Periodic Acid Modification of Chemical-Bath Deposited SnO2 Electron Transport Layers for Perovskite Solar Cells and Mini Modules.: This paper presents a methodology for the modification of SnO2 electron transport layers, used to increase the efficiency of perovskite solar cells and mini-modules (Lin H et al., 2023).
- Zwitterion-Functionalized SnO2 Substrate Induced Sequential Deposition of Black-Phase FAPbI3 with Rearranged PbI2 Residue.: Research on enhancing the deposition of black-phase FAPbI3 on zwitterion-functionalized SnO2 substrates, focusing on perovskite solar cell improvements (Zhao Y et al., 2022).
- Improved stability and efficiency of polymer-based selenium solar cells through the usage of tin(iv) oxide in the electron transport layers and the analysis of aging dynamics.: The study investigates the role of tin(IV) oxide in enhancing the stability and efficiency of polymer-based selenium solar cells (Zhang Q et al., 2020).
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Li-Ping Li et al.
Chemical communications (Cambridge, England), 49(17), 1762-1764 (2013-01-25)
ZnSn(OH)(6) and binary-component SnO(2)-ZnSn(OH)(6) were introduced as affinity probes for phosphopeptide enrichment for the first time. Two strategies, either ZnSn(OH)(6) and SnO(2) serial enrichment or binary-component SnO(2)-ZnSn(OH)(6) enrichment in a single run, were proposed to enhance multi-phosphopeptide enrichment and to
Dawei Su et al.
Chemical communications (Cambridge, England), 49(30), 3131-3133 (2013-03-13)
An in situ hydrothermal synthesis approach has been developed to prepare SnO2@graphene nanocomposites. The nanocomposites exhibited a high reversible sodium storage capacity of above 700 mA h g(-1) and excellent cyclability for Na-ion batteries. In particular, they also demonstrated a
Junfei Liang et al.
ACS applied materials & interfaces, 4(11), 5742-5748 (2012-10-24)
A flexible free-standing graphene/SnO₂ nanocomposites paper (GSP) was prepared by coupling a simple filtration method and a thermal reduction together for the first time. Compared with the pure SnO₂ nanoparticles, the GSP exhibited a better cycling stability, because the graphene
Lina Gao et al.
Langmuir : the ACS journal of surfaces and colloids, 29(3), 957-964 (2012-12-25)
As advanced electrodes for direct alcohol fuel cells, graphene-Pd and graphene-Pt composites with a trace of SnO(2) have been successfully synthesized by a modified electroless plating technique. The surface of graphene oxide is first sensitized by Sn(2+) ions, and subsequently
Guangmin Zhou et al.
Nanoscale, 5(4), 1576-1582 (2013-01-19)
We explore a hybrid material consisting of SnO(2) nanoparticles (NPs) embedded in the porous shells of carbon cages (SnO(2)-PSCC). The hybrid material exhibits improved kinetics of lithiation-delithiation and high reversible capacity, and excellent cyclic stability without capacity loss over 100
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service