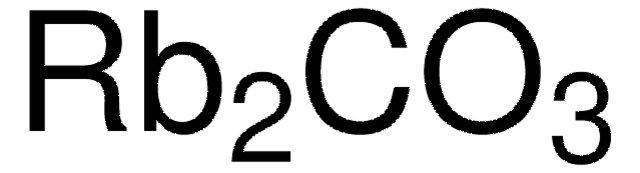All Photos(1)
About This Item
Empirical Formula (Hill Notation):
Rb
CAS Number:
Molecular Weight:
85.47
EC Number:
MDL number:
UNSPSC Code:
11101711
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.6% trace metals basis
form
ingot
reaction suitability
reagent type: reductant
packaging
pkg of Packaged in: Breakseal Ampule
resistivity
11.0 μΩ-cm, 20°C
impurities
0.2-0.4% Cs
bp
686 °C (lit.)
mp
38-39 °C (lit.)
density
1.53 g/mL at 25 °C (lit.)
SMILES string
[Rb]
InChI
1S/Rb
InChI key
IGLNJRXAVVLDKE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Rubidium is a highly electropositive alkaline metal that can be obtained as a byproduct of refining lithium from lepidolite. It is used in photomultiplier tubes, laser cooling, atomic clock, photocell, nuclear medicine, and specialty glass.
Application
Rubidium can be used as a starting material to synthesize rubidium hydrazinidoborane (RbN2H3BH3).
It can also be used as a reducing agent to synthesize reduced polycyclic aromatic hydrocarbons.
It can also be used as a reducing agent to synthesize reduced polycyclic aromatic hydrocarbons.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nan-Nan Bu et al.
Biosensors & bioelectronics, 43, 200-204 (2013-01-15)
Restricted target accessibility and surface-induced perturbation of the aptamer structure are the main limitations in single-stranded DNA aptamer-based electrochemical sensors. Chemical labeling of the aptamer with a probe at the end of aptamer is inefficient and time-consuming. In this work
Kazuhiro Abe et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(45), 18401-18406 (2012-10-24)
Gastric H(+),K(+)-ATPase is responsible for gastric acid secretion. ATP-driven H(+) uptake into the stomach is efficiently accomplished by the exchange of an equal amount of K(+), resulting in a luminal pH close to 1. Because of the limited free energy
Takashi Ikeda et al.
The Journal of chemical physics, 137(4), 041101-041101 (2012-08-03)
Hydration structure and polarization of Rb(+) and Cs(+) in liquid water at ambient conditions were studied by first principles molecular dynamics. Our systematic analysis of the relevant electronic structures, based on maximally localized Wannier functions, revealed that the dipole moment
Silvano Lizzit et al.
Nano letters, 12(9), 4503-4507 (2012-08-09)
High-quality, large-area epitaxial graphene can be grown on metal surfaces, but its transport properties cannot be exploited because the electrical conduction is dominated by the substrate. Here we insulate epitaxial graphene on Ru(0001) by a stepwise intercalation of silicon and
Marco Papagno et al.
ACS nano, 6(10), 9299-9304 (2012-10-02)
By combining angle-resolved photoemission spectroscopy and scanning tunneling microscopy we reveal the structural and electronic properties of multilayer graphene on Ru(0001). We prove that large ethylene exposure allows the synthesis of two distinct phases of bilayer graphene with different properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service