479896
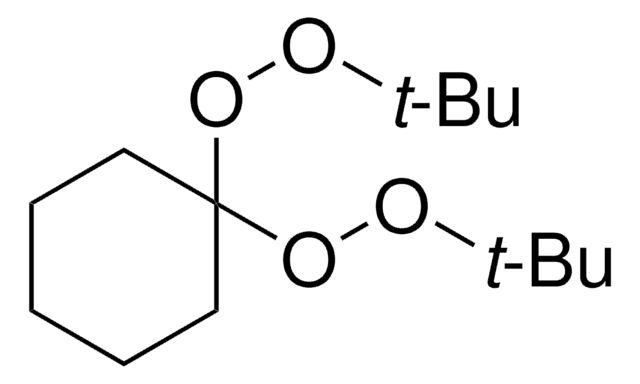
Luperox® 231, 1,1-Bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane
92%
Synonym(s):
1,1-Bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3COO]2C6H7(CH3)3
CAS Number:
Molecular Weight:
302.45
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
Recommended Products
Quality Level
Assay
92%
refractive index
n20/D 1.441 (lit.)
mp
≥50 °C (SADT) (lit.)
density
0.904 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC1CC(C)(C)CC(C1)(OOC(C)(C)C)OOC(C)(C)C
InChI
1S/C17H34O4/c1-13-10-16(8,9)12-17(11-13,20-18-14(2,3)4)21-19-15(5,6)7/h13H,10-12H2,1-9H3
InChI key
NALFRYPTRXKZPN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Polymerization Initiator
Legal Information
Product of Arkema Inc.
Luperox is a registered trademark of Arkema Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Org. Perox. B
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Mitsui et al.
Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences, (110)(110), 42-48 (1992-01-01)
A 13-week subchronic toxicity study of 1,1-bis(t-butylperoxy)3,3,5-trimethyl cyclohexane (TMCH) was performed in male and female B6C3F1 mice by feeding a CRF-1 powder diet containing 0, 0.5, 1.0, 2.0 and 4.0% TMCH, to determine the maximum tolerable dose (MTD) for subsequent
Xiaopeng Shan et al.
Proceedings of the National Academy of Sciences of the United States of America, 102(15), 5340-5345 (2005-04-02)
The reaction of [Fe(2)(mu-OH)(2)(6-Me(3)-TPA)(2)](2+) (1) [6-Me(3)-TPA, Tris(6-methyl-2-pyridylmethyl)amine] with O(2) in CH(2)Cl(2) at -80 degrees C gives rise to two new intermediates, 2 and 3, before the formation of previously characterized [Fe(2)(O)(O(2))(6-Me(3)-TPA)(2)](2+) (4) that allow the oxygenation reaction to be monitored
M Mitsui et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 31(12), 929-933 (1993-12-01)
1,1-Bis(tert-butylperoxy)-3.3.5-trimethylcyclohexane (BBTC) is widely used in the manufacture of rubber. The present carcinogenicity study in B6C3F1 mice was carried out in order to assess its potential to induce tumours. BBTC was administered at dietary levels of 0 (control), 0.25 and
Jo-Ming Tseng et al.
Journal of hazardous materials, 192(3), 1427-1436 (2011-07-26)
Over the past 30 years, the field of thermal analysis of organic peroxides has become an important issue in chemical engineering departments, safety departments, and in companies working with polymerization, petrifaction process, and so on. The contributions of thermal analysis
V S Trubetskoy et al.
Bioconjugate chemistry, 10(4), 624-628 (1999-07-20)
The assembly of DNA into compact particles that do not aggregate in physiologic salt solution occurs naturally in chromatin and viral particles but has been challenging to duplicate using artificial constructs. Cross-linking amino-containing polycations in the presence of DNA with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Bicyclo[2.2.1]hepta-2,5-diene 98%](/deepweb/assets/sigmaaldrich/product/structures/304/819/dfa7c176-c370-4fb5-acf1-28d751241a50/640/dfa7c176-c370-4fb5-acf1-28d751241a50.png)





