All Photos(2)
About This Item
Empirical Formula (Hill Notation):
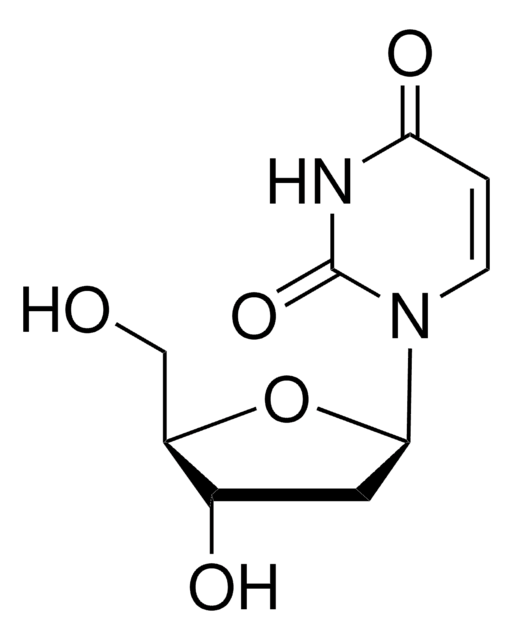
C10H14N2O6
CAS Number:
Molecular Weight:
258.23
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
183-184 °C (lit.)
SMILES string
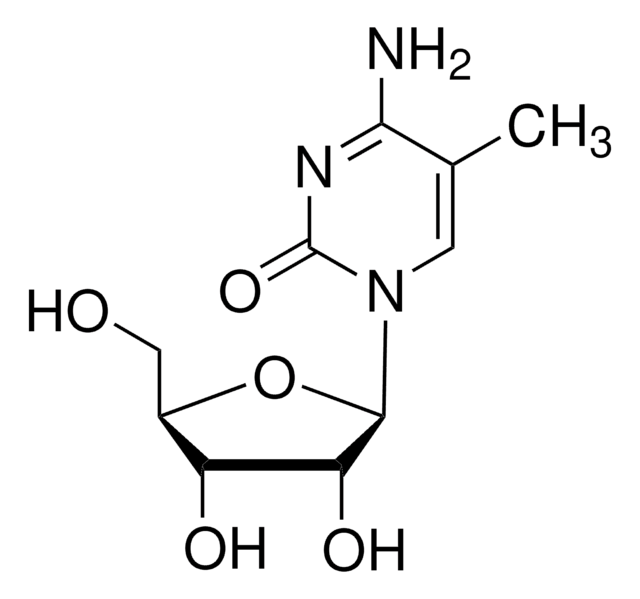
CC1=CN([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)C(=O)NC1=O
InChI
1S/C10H14N2O6/c1-4-2-12(10(17)11-8(4)16)9-7(15)6(14)5(3-13)18-9/h2,5-7,9,13-15H,3H2,1H3,(H,11,16,17)/t5-,6-,7-,9-/m1/s1
InChI key
DWRXFEITVBNRMK-JXOAFFINSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H F Becker et al.
Journal of molecular biology, 274(4), 505-518 (1998-01-07)
Almost all transfer RNA molecules sequenced so far contain two universal modified nucleosides at positions 54 and 55, respectively: ribothymidine (T54) and pseudouridine (psi 55). To identify the tRNA elements recognized by tRNA:m5uridine-54 methyltransferase and tRNA:pseudouridine-55 synthase from the yeast
Benoit Desmolaize et al.
Nucleic acids research, 39(21), 9368-9375 (2011-08-10)
Methyltransferases that use S-adenosylmethionine (AdoMet) as a cofactor to catalyse 5-methyl uridine (m(5)U) formation in tRNAs and rRNAs are widespread in Bacteria and Eukaryota, and are also found in certain Archaea. These enzymes belong to the COG2265 cluster, and the
Gregory E R Gordon et al.
Journal of biotechnology, 151(1), 108-113 (2010-11-30)
This paper describes a high yielding coupled enzymatic reaction using Bacillus halodurans purine nucleoside phosphorylase (PNP) and E. coli uridine phosphorylase (UP) for synthesis of 5-methyluridine (5-MU) by transglycosylation. Key parameters such as reaction temperature, pH, reactant loading, reactor configuration
Pseudouridine and ribothymidine formation in the tRNA-like domain of turnip yellow mosaic virus RNA.
H F Becker et al.
Nucleic acids research, 26(17), 3991-3997 (1998-08-15)
The last 82 nucleotides of the 6.3 kb genomic RNA of plant turnip yellow mosaic virus (TYMV), the so-called 'tRNA-like' domain, presents functional, structural and primary sequence homologies with canonical tRNAs. In particular, one of the stem-loops resembles the TPsi(pseudouridine)-branch
Marino J E Resendiz et al.
Journal of the American Chemical Society, 134(30), 12478-12481 (2012-07-26)
Photolabile nucleotides that disrupt nucleic acid structure are useful mechanistic probes and can be used as tools for regulating biochemical processes. Previous probes can be limited by the need to incorporate multiple modified nucleotides into oligonucleotides and in kinetic studies
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service