All Photos(1)
About This Item
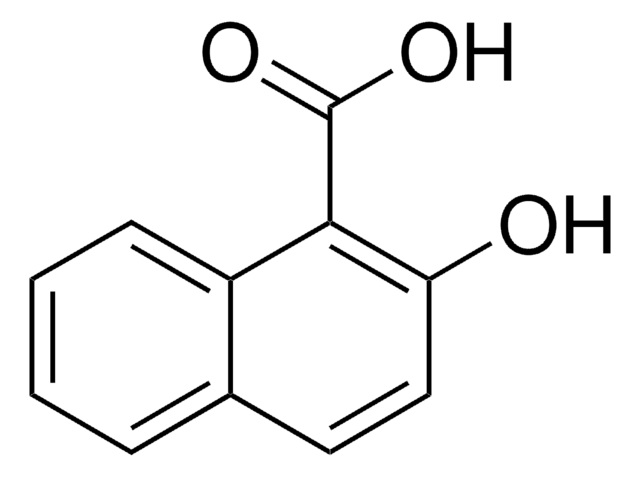
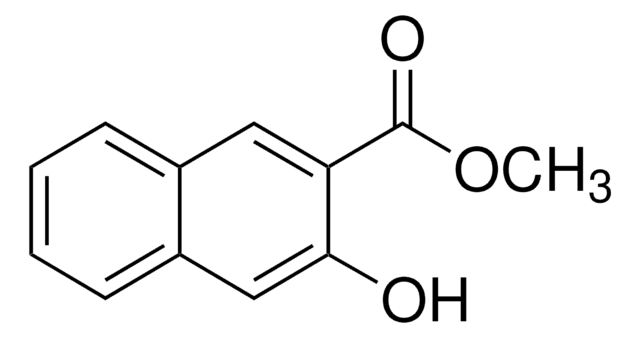
Linear Formula:
HOC10H6CO2CH3
CAS Number:
Molecular Weight:
202.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
76-80 °C (lit.)
SMILES string
COC(=O)c1ccc2ccccc2c1O
InChI
1S/C12H10O3/c1-15-12(14)10-7-6-8-4-2-3-5-9(8)11(10)13/h2-7,13H,1H3
InChI key
HMIBDRSTVGFJPB-UHFFFAOYSA-N
General description
The asymmetric unit of the crystal of methyl 1-hydroxy-2-naphthoate contains two independent planar molecules that exhibit intramolecular hydrogen bonds. Methyl 1-hydroxy-2-naphthoate can be prepared from 1-hydroxy-2-naphthoic acid, via esterification.
Application
Methyl 1-hydroxy-2-naphthoate may be used as a starting reagent for the synthesis of axially chiral benzimidazole derivatives.
It may also be employed in the synthesis of the following aza-mollugin derivatives:
It may also be employed in the synthesis of the following aza-mollugin derivatives:
- azamollugin
- 2-desmethyl-azamollugin
- 2,2-didesmethyl-azamollugin
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hitomi Nishino et al.
Bioorganic & medicinal chemistry letters, 26(2), 524-525 (2015-12-19)
Oxomollugin (2) is a degradation product of mollugin (1) and a potent inhibitor of NO-production including nuclear factor kappa B signals. In our endeavor to develop a potent anti-inflammatory compound, we synthesized several aza-derivatives of oxomollugin (2) and evaluated their
Effects of electronic structures on the excited-state intramolecular proton transfer of 1-hydroxy-2-acetonaphthone and related compounds.
Tobita S, et al.
The Journal of Physical Chemistry A, 102(27), 5206-5214 (1998)
Feijun Wang et al.
Beilstein journal of organic chemistry, 8, 726-731 (2012-09-28)
Axially chiral oxazoline-carbene ligands with an N-naphthyl framework were successfully prepared, and their coordination behavior with AuCl·SMe(2) was also investigated, affording the corresponding Au(I) complexes in moderate to high yields.
Methyl 1-hydroxy-2-naphthoate.
Jin LF and Xiao FP.
Acta Crystallographica Section E, Structure Reports Online, 61(5), o1520-o1522 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service