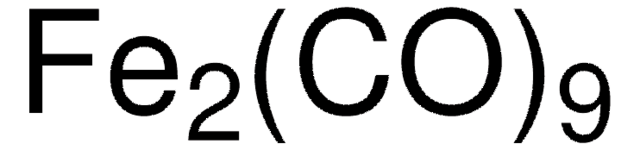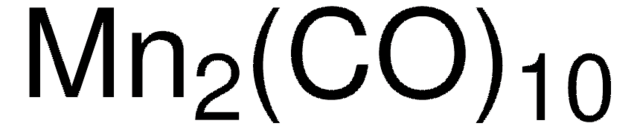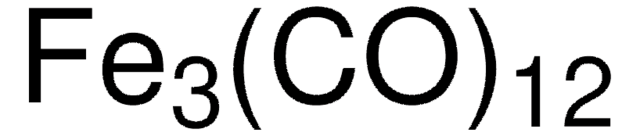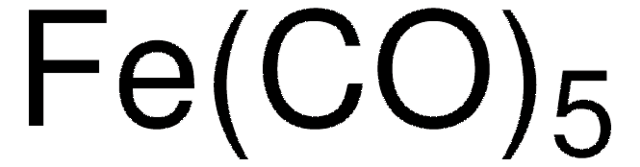60811
Cobalt carbonyl
moistened with hexane (hexane 1-10%), ≥90% (Co)
Synonym(s):
Cobalt tetracarbonyl dimer, Di-μ-carbonylhexacarbonyldicobal, Octacarbonyldicobalt, Dicobalt octacarbonyl
About This Item
Recommended Products
Assay
≥90% (Co)
form
solid
quality
moistened with hexane (hexane 1-10%)
reaction suitability
core: cobalt
reagent type: catalyst
storage temp.
2-8°C
SMILES string
[Co++].[Co++].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+]
InChI
1S/8CO.2Co/c8*1-2;;/q;;;;;;;;2*+2
InChI key
MQIKJSYMMJWAMP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Cobalt carbonyl [Co2(CO)8] is commonly used as a catalyst in the hydroformylation (oxo reaction) of alkenes.
- Along with pyridine, it can be used as a catalyst in the carboxylation of alkenes into corresponding acids and esters.
- It is employed as a key precursor in the preparation of cobalt platinum (CoPt3), cobalt sulfide (Co3S4) and cobalt selenide (CoSe2) nanocrystals.
- It is also used as a reagent in Pauson-Khand cyclizations and Nicholas reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Repr. 2 - Self-heat. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Inhalation
Target Organs
Nervous system
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
-9.4 °F
Flash Point(C)
-23 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Ultrasonic spray pyrolysis produces scalable nanomaterials like metal oxides and quantum dots for diverse applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service