69723
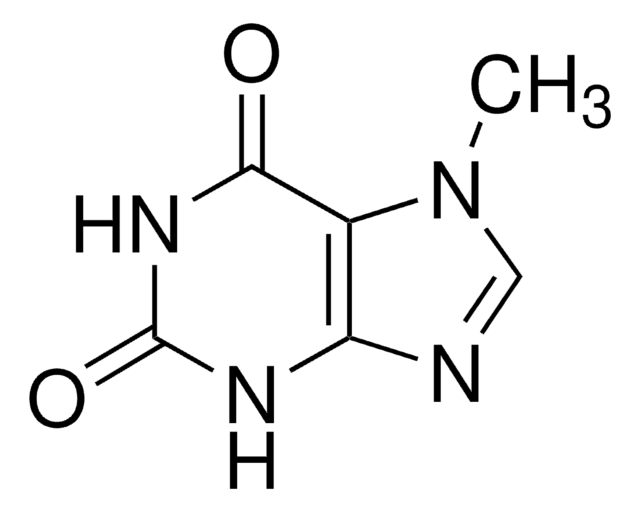
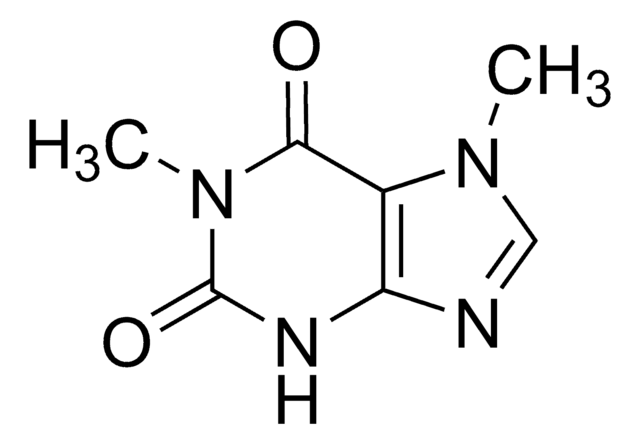
7-Methylxanthine
≥98.0% (HPLC)
Synonym(s):
2,6-Dihydroxy-7-methylpurine, Heteroxanthine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N4O2
CAS Number:
Molecular Weight:
166.14
Beilstein:
171027
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (HPLC)
form
powder
mp
≥300 °C
SMILES string
Cn1cnc2NC(=O)NC(=O)c12
InChI
1S/C6H6N4O2/c1-10-2-7-4-3(10)5(11)9-6(12)8-4/h2H,1H3,(H2,8,9,11,12)
InChI key
PFWLFWPASULGAN-UHFFFAOYSA-N
Gene Information
rat ... Adora1(29290) , Adora2a(25369)
Looking for similar products? Visit Product Comparison Guide
General description
7-Methylxanthine is an oxopurine, which belongs to the class of xanthines. It may be synthesized by the reaction between 4-amino-1-methylimidazole-5-carboxamide and diethyl carbonate.
Application
7-Methylxanthine can be used as a building block to synthesize tricyclic imidazo[2,1-i]purinone derivatives as potential adenosine receptor antagonists.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
related product
Product No.
Description
Pricing
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K Trier et al.
The British journal of ophthalmology, 83(12), 1370-1375 (1999-11-27)
To examine a possible effect of 7-methylxanthine, theobromine, acetazolamide, or L-ornithine on the ultrastructure and biochemical composition of rabbit sclera. Groups of pigmented rabbits, six in each group, were dosed during 10 weeks with one of the substances under investigation
Swati Sucharita Dash et al.
Current microbiology, 55(1), 56-60 (2007-06-08)
In this study, the kinetics of degradation of caffeine and related methylxanthines by induced cells of Pseudomonas sp. was performed. The kinetics data showed that degradation of caffeine, theobromine, and 7-methylxanthine followed Michealis-Menten kinetics. The values of K (m) are
Dongmei Cui et al.
Acta ophthalmologica, 89(4), 328-334 (2009-10-29)
The aim of this study was to determine the effect of the adenosine receptor antagonist 7-methylxanthine (7-MX) on form deprivation myopia in 3-week-old guinea pigs. Two groups of 3-week-old guinea pigs were subjected to monocular deprivation (MD) using a diffuser
Kouichi Mizuno et al.
FEBS letters, 534(1-3), 75-81 (2003-01-16)
In coffee and tea plants, caffeine is synthesized from xanthosine via a pathway that includes three methylation steps. We report the isolation of a bifunctional coffee caffeine synthase (CCS1) clone from coffee endosperm by reverse transcription-polymerase chain reaction (RT-PCR) and
W A Tramontano et al.
Phytochemistry, 29(1), 31-33 (1990-01-01)
The use of a DNA alkylating agent, which induces poly(ADP-ribose) formation, has been employed to study the incorporation of [adenine 14C]NAD into pea root meristem nuclei, which is a prerequisite for poly(ADP-ribose) synthesis. The incorporation of [adenine 14C]NAD is significantly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service