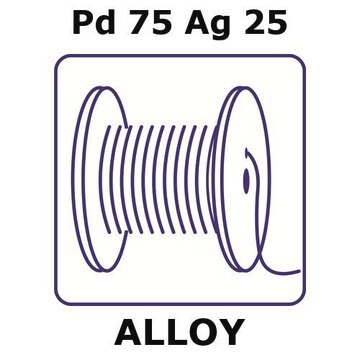
GF65598171
Palladium
wire reel, 0.2m, diameter 0.25mm, as drawn, 99.99+%
Synonym(s):
Palladium, PD005140
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Pd
CAS Number:
Molecular Weight:
106.42
MDL number:
UNSPSC Code:
12141733
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.5%
form
wire
manufacturer/tradename
Goodfellow 655-981-71
resistivity
9.96 μΩ-cm, 20°C
L × diam.
0.2 m × 0.25 mm
bp
2970 °C (lit.)
mp
1554 °C (lit.)
density
12.02 g/cm3 (lit.)
SMILES string
[Pd]
InChI
1S/Pd
InChI key
KDLHZDBZIXYQEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
For updated SDS information please visit www.goodfellow.com.
Legal Information
Product of Goodfellow
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pazhamalai Anbarasan et al.
Chemical Society reviews, 40(10), 5049-5067 (2011-04-30)
The palladium-catalyzed cyanation of Ar-X (X = I, Br, Cl, OTf, and H) allows for an efficient access towards benzonitriles. After its discovery in 1973 and following significant improvements in recent decades, this methodology has become nowadays the most popular
Qing-An Chen et al.
Chemical Society reviews, 42(2), 497-511 (2012-11-10)
The transition metal catalyzed asymmetric hydrogenation of unsaturated compounds arguably presents one of the most attractive methods for the synthesis of chiral compounds. Over the last few decades, Pd has gradually grown up as a new and popular metal catalyst
Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications.
Richard I McDonald et al.
Chemical reviews, 111(4), 2981-3019 (2011-03-25)
Xiao-Feng Wu et al.
ChemSusChem, 6(2), 229-241 (2013-01-12)
Palladium-catalyzed coupling reactions have become a powerful tool for advanced organic synthesis. This type of reaction is of significant value for the preparation of pharmaceuticals, agrochemicals, as well as advanced materials. Both, academic as well as industrial laboratories continuously investigate
Masahiro Yoshida
Chemical & pharmaceutical bulletin, 60(3), 285-299 (2012-03-03)
It is known that propargylic compounds having an ester and a halide at the propargylic positions react with palladium complexes leading to π-propargylpalladium and allenylpalladium complexes, which cause various transformations in the presence of the reactants. The aim of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service