All Photos(3)
About This Item
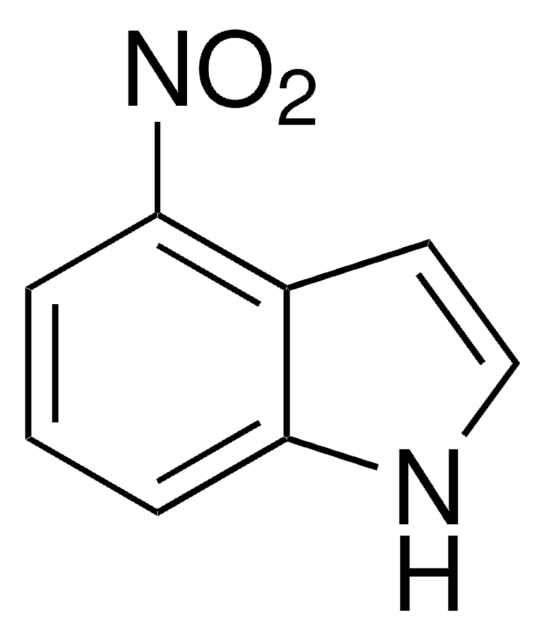
Empirical Formula (Hill Notation):
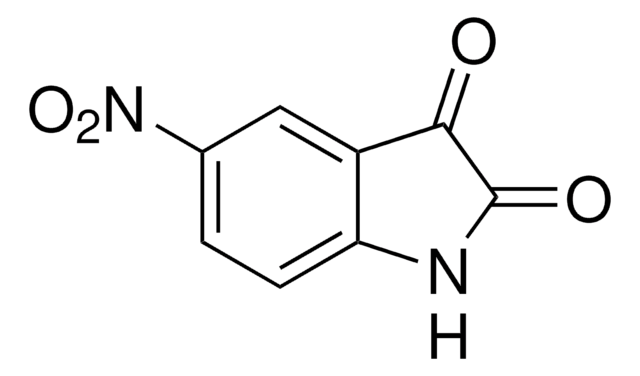
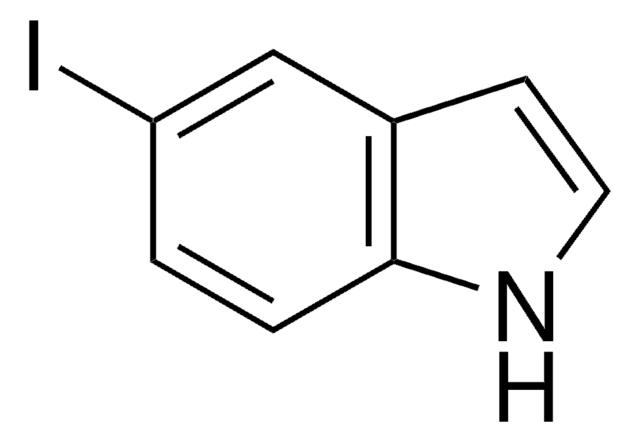
C8H6N2O2
CAS Number:
Molecular Weight:
162.15
Beilstein:
383777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
140-142 °C (lit.)
SMILES string
[O-][N+](=O)c1ccc2[nH]ccc2c1
InChI
1S/C8H6N2O2/c11-10(12)7-1-2-8-6(5-7)3-4-9-8/h1-5,9H
InChI key
OZFPSOBLQZPIAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Protein Kinase Inhibitors and antiproliferative agents
- Positive Allosteric Modulators of Metabotropic Glutamate Receptor 4 (mGlu4)
- Antifungal agents
- Cannabinoid receptor type 1 (CB1) antagonists
- Potential anticancer agents
- Potential antivascular agents
- Selective Anti-leukemic agents
- Anti human immunodeficiency virus subtype 1 (HIV-1) agents
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wengen Ouyang et al.
Journal of chemical theory and computation, 16(1), 666-676 (2019-12-10)
The importance of many-body dispersion effects in layered materials subjected to high external loads is evaluated. State-of-the-art many-body dispersion density functional theory calculations performed for graphite, hexagonal boron nitride, and their heterostructures were used to fit the parameters of a
D Loakes et al.
Journal of molecular biology, 270(3), 426-435 (1997-07-18)
The nature of DNA containing the deoxyribosyl derivative of 5-nitroindole has been investigated. 5-Nitroindole has been shown to give good stability when present in duplexes opposite natural bases, with only slightly reduced melting temperatures. However, enhanced stability occurs when it
M Dubin et al.
Biochemical pharmacology, 48(7), 1483-1492 (1994-10-07)
5-Nitroindole (NI), a mutagenic nitroarene, was assayed for cytotoxic effects on rat hepatocytes. After incubation with 25-100 microM NI, the adenylate energy charge of the hepatocytes decreased significantly as a result of the decrease in ATP and the increase in
David Loakes et al.
Journal of the American Chemical Society, 131(41), 14827-14837 (2009-09-26)
Hydrophobic base analogues (HBAs) have shown great promise for the expansion of the chemical and coding potential of nucleic acids but are generally poor polymerase substrates. While extensive synthetic efforts have yielded examples of HBAs with favorable substrate properties, their
Xuemei Zhang et al.
Biochemistry, 49(14), 3009-3023 (2010-03-02)
Most models accounting for the efficiency and fidelity of DNA polymerization invoke the use of either hydrogen bonding contacts or complementarity of shape and size between the formed base pair. This report evaluates these mechanisms by quantifying the ability of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service