29240
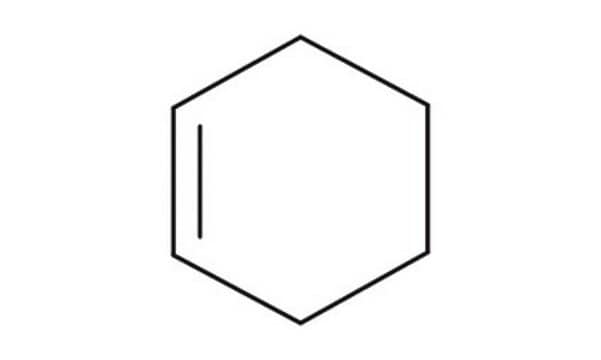
Cyclohexene
contains 100 ppm BHT as inhibitor, ≥99.0%
Synonym(s):
Tetrahydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10
CAS Number:
Molecular Weight:
82.14
Beilstein:
906737
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
2.8 (vs air)
Quality Level
vapor pressure
160 mmHg ( 20 °C)
Assay
≥99.0%
autoignition temp.
590 °F
contains
100 ppm BHT as inhibitor
expl. lim.
5 %
evapn. residue
≤0.01%
refractive index
n20/D 1.446 (lit.)
n20/D 1.446
bp
83 °C (lit.)
mp
−104 °C (lit.)
density
0.811 g/mL at 25 °C (lit.)
SMILES string
C1CCC=CC1
InChI
1S/C6H10/c1-2-4-6-5-3-1/h1-2H,3-6H2
InChI key
HGCIXCUEYOPUTN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclohexene has been used in the green synthesis of cyclohexene oxide via hydrogen peroxide epoxidation in glycerol-based solvents using bis[3,5-bis(trifluoromethyl)-diphenyl] diselenide as a precatalyst. It can also undergo hydrogen peroxide oxidation in the presence of peroxytungstate-oxalic acid complex catalyst to form adipic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Epoxidation of cyclooctene and cyclohexene with hydrogen peroxide catalyzed by bis [3, 5-bis (trifluoromethyl)-diphenyl] diselenide: Recyclable catalyst-containing phases through the use of glycerol-derived solvents
Garcia-Marin H, et al.
J. Mol. Catal. A: Chem., 334(1), 83-88 (2011)
Catalytic hydrogenation of cyclohexene: 3. Gas-phase and liquid-phase reaction on supported palladium.
Gonzo EE and Boudart M.
J. Catal., 52(3), 462-471 (1978)
Aerobic oxidative Heck/dehydrogenation reactions of cyclohexenones: efficient access to meta-substituted phenols.
Yusuke Izawa et al.
Angewandte Chemie (International ed. in English), 52(13), 3672-3675 (2013-02-21)
Clean synthesis of adipic acid by direct oxidation of cyclohexene with H2O2 over peroxytungstate-organic complex catalysts.
Deng Y, et al.
Green Chemistry, 1(6), 275-276 (1999)
Oleg Y Lyakin et al.
Inorganic chemistry, 50(12), 5526-5538 (2011-05-24)
Complexes [(BPMEN)Fe(II)(CH(3)CN)(2)](ClO(4))(2) (1, BPMEN = N,N'-dimethyl-N,N'-bis(2-pyridylmethyl)-1,2-diaminoethane) and [(TPA)Fe(II)(CH(3)CN)(2)](ClO(4))(2) (2, TPA = tris(2-pyridylmethyl)amine) are among the best nonheme iron-based catalysts for bioinspired oxidation of hydrocarbons. Using EPR and (1)H and (2)H NMR spectroscopy, the iron-oxygen intermediates formed in the catalyst systems
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service