All Photos(3)
About This Item
Empirical Formula (Hill Notation):
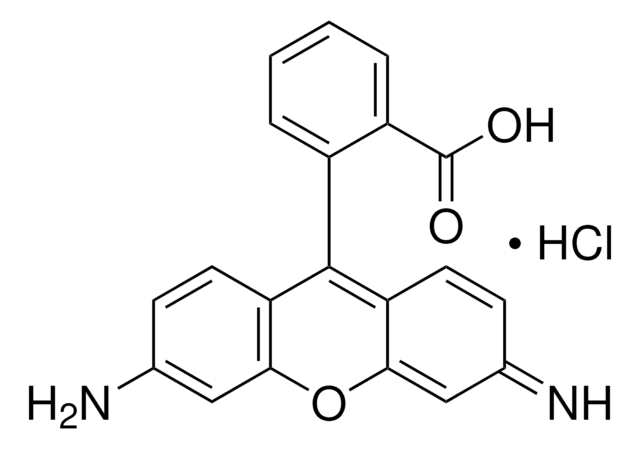
C20H14N2O3 · HCl
CAS Number:
Molecular Weight:
366.80
Beilstein:
4631860
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
form
powder or crystals
Quality Level
composition
Dye content, ≥75%
mp
>300 °C (lit.)
λmax
496 nm
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Cl.Nc1ccc2c(OC3=CC(=N)C=CC3=C2c4ccccc4C(O)=O)c1
InChI
1S/C20H14N2O3.ClH/c21-11-5-7-15-17(9-11)25-18-10-12(22)6-8-16(18)19(15)13-3-1-2-4-14(13)20(23)24;/h1-10,21H,22H2,(H,23,24);1H
InChI key
JNGRENQDBKMCCR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Rhodamine 110 chloride (R110) is a laser grade dye.
Application
Rhodamine 110 chloride has been used for the synthesis of the rhodamine 110 octadecyl ester (R110C18).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sambashiva Banala et al.
ACS chemical biology, 7(2), 289-293 (2011-10-27)
A caged rhodamine 110 derivative for the specific labeling of SNAP-tag fusion proteins is introduced. The caged rhodamine 110 derivative permits the labeling of cell surface proteins in living cells and of intracellular proteins in fixed cells. The probe requires
Jaime G Mayoral et al.
Applied biochemistry and biotechnology, 160(1), 1-8 (2009-01-28)
A novel end-point fluorimetric procedure based on the use of rhodamine-110-labeled specific substrate was developed to determine trypsin activities in biological samples. We evaluated the ability of trichloroacetic acid and acetic acid to stop the enzymatic reaction without hindering the
Aya Shibata et al.
Bioorganic & medicinal chemistry letters, 18(7), 2246-2249 (2008-03-25)
We have developed a new fluorescent probe for biological thiol. The probe was synthesized by the modification of the 2,4-dinitrobenzenesulfonyl group with rhodamine 110. The selective detection of thiol species such as cysteine or glutathione was achieved in biological conditions.
Melissa M Yatzeck et al.
Bioorganic & medicinal chemistry letters, 18(22), 5864-5866 (2008-07-04)
A derivative of rhodamine 110 has been designed and assessed as a probe for cytochrome P450 activity. This probe is the first to utilize a 'trimethyl lock' that is triggered by cleavage of an ether bond. In vitro, fluorescence was
Michelle M Martinez et al.
Analytical and bioanalytical chemistry, 396(3), 1177-1185 (2009-11-26)
Early detection of apoptotic cells via caspase activity is demonstrated with fast response time. Fluorescence correlation spectroscopy (FCS) is used to identify the presence of a cleaved fluorogenic probe based on the fluorescence of rhodamine 110 in Jurkat cells. FCS
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service