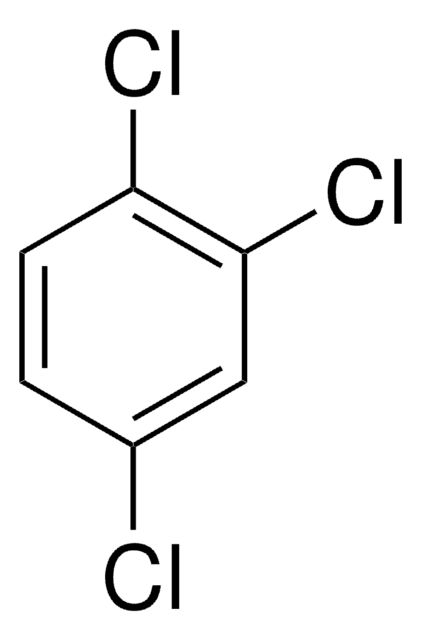
About This Item
UV/Vis spectroscopy: suitable
Recommended Products
vapor density
>6 (vs air)
Quality Level
vapor pressure
1 mmHg ( 40 °C)
Assay
≥99%
form
solid or liquid
autoignition temp.
1060 °F
expl. lim.
6.6 %, 150 °F
technique(s)
HPLC: suitable
UV/Vis spectroscopy: suitable
impurities
<0.020% water
refractive index
n20/D 1.571 (lit.)
bp
214 °C (lit.)
mp
16 °C (lit.)
solubility
water: insoluble
density
1.454 g/mL at 25 °C (lit.)
λ
H2O reference
UV absorption
λ: 308 nm Amax: 1.00
λ: 310 nm Amax: 0.50
λ: 350 nm Amax: 0.05
λ: 375-400 nm Amax: 0.01
application(s)
food and beverages
SMILES string
Clc1ccc(Cl)c(Cl)c1
InChI
1S/C6H3Cl3/c7-4-1-2-5(8)6(9)3-4/h1-3H
InChI key
PBKONEOXTCPAFI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Application of electrospun polyacrylonitrile/Zn-MOF-74@GO nanocomposite as the sorbent for online micro solid-phase extraction of chlorobenzenes in water, soil, and food samples prior to liquid chromatography analysis: This article explores the use of an electrospun polyacrylonitrile/Zn-MOF-74@GO nanocomposite for the extraction of chlorobenzenes, including 1,2,4-Trichlorobenzene, from various samples. The method shows high efficiency and selectivity, making it suitable for environmental and food safety applications (Amini et al., 2021).
- Facile synthesis and immobilization of functionalized covalent organic framework-1 for electrochromatographic separation: This research involves the synthesis and application of a functionalized covalent organic framework for the electrochromatographic separation of various compounds, including chlorobenzenes like 1,2,4-Trichlorobenzene. The method shows potential for analytical and separation sciences (Bao et al., 2021).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113.0 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service