4370285
Trypsin, TPCK-Treated
Synonym(s):
Trypsin, TPCK treated for Cell Culture
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
shipped in
dry ice
storage temp.
−20°C
Related Categories
General description
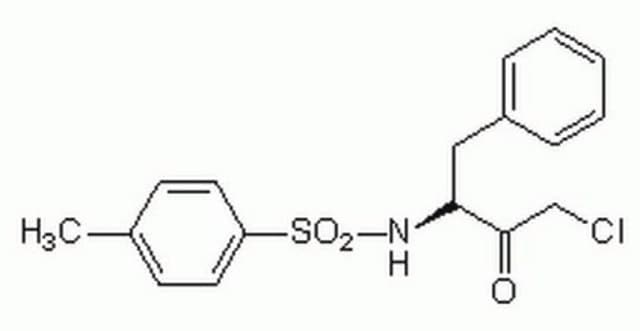
Trypsin is a serine protease that specifically hydrolyzes peptide bonds at the carboxyl side of lysine and arginine residues. This modified trypsin has been treated with N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) to inactivate extraneous chymotryptic activity. Each package contains 8 vials, with 25 μg in each vial.
Application
Trypsin, TPCK-Treated has been used:
- as a supplement in Dulbecco′s Modified Eagle Medium (DMEM) for porcine delta coronavirus (PDCoV) infection experiments using epithelial-like pig kidney cell line (LLC-PK1)
- to detach the human umbilical vein endothelial cells (HUVEC) for annexin-V/propidium Iodide (PI) staining assay
- in minimum essential medium (MEM) for multicycle replication kinetics
Biochem/physiol Actions
N-p-Tosyl-L-phenylalanine chloromethyl ketone (TPCK) serves as an irreversible inhibitor of chymotrypsin. Trypsin induces human fibrocyte differentiation. Trypsin is widely used in proteomics for protein sample digestion. In cell culture, trypsinization is carried out to dislodge adherent cells from each other and the walls of the culture vessel.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zdeněk Perutka et al.
Molecules (Basel, Switzerland), 23(10) (2018-10-17)
Trypsin is the protease of choice for protein sample digestion in proteomics. The most typical active forms are the single-chain β-trypsin and the two-chain α-trypsin, which is produced by a limited autolysis of β-trypsin. An additional intra-chain split leads to
Michael J V White et al.
PloS one, 8(8), e70795-e70795 (2013-08-21)
Trypsin-containing topical treatments can be used to speed wound healing, although the mechanism of action is unknown. To help form granulation tissue and heal wounds, monocytes leave the circulation, enter the wound tissue, and differentiate into fibroblast-like cells called fibrocytes.
Zhonghui Ling et al.
Reproductive biology, 21(2), 100483-100483 (2021-02-26)
Vascular endothelial cell damage is regarded as the carrier in the progression of the pathological changes of preeclampsia (PE) from the placenta to maternal organs. MicroRNA (miR)-141-3p was aberrantly expressed during PE pathogenesis. We investigated the role of miR-141-3p in
Yu Bai et al.
Microbial pathogenesis, 150, 104645-104645 (2020-12-08)
Influenza virus is responsible for significant morbidity and mortality worldwide. Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is the major cause of death in influenza virus infected patients. Recent studies indicated that active glucagon like peptide-1 (GLP-1) encoded by
Jian-Ping Dai et al.
International journal of molecular sciences, 19(4) (2018-03-24)
Oxymatrine (OMT) is a strong immunosuppressive agent that has been used in the clinic for many years. In the present study, by using plaque inhibition, luciferase reporter plasmids, qRT-PCR, western blotting, and ELISA assays, we have investigated the effect and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service