D2629
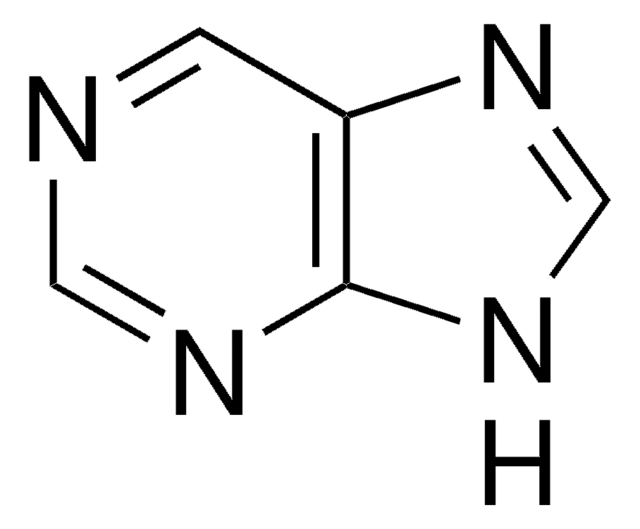
6-(Dimethylamino)purine
≥98%
Synonym(s):
6-DMAP, N6,N6-Dimethyladenine
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C7H9N5
CAS Number:
Molecular Weight:
163.18
Beilstein:
7634
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98%
form
powder
mp
259-262 °C (lit.)
solubility
water: 50 mg/mL, clear to hazy, colorless to light yellow
storage temp.
−20°C
SMILES string
CN(C)c1ncnc2[nH]cnc12
InChI
1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11)
InChI key
BVIAOQMSVZHOJM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-(Dimethylamino)purine (6-DMAP) is a purine-based metabolite with two condensed heterocyclic rings and two methyl groups linked to the amino group of the purine unit of adenine.
Application
6-(Dimethylamino)purine has been used:
- as a supplement in GR-1 aa medium (bovine medium) for parthenogenetic activation of bovine oocytes to study its potential for embryo development
- in the activation step during the production of nuclear transfer embryos
- as a supplement in HCR2aa medium to activate interspecies embryos derived from interspecies somatic cell nuclear transfer (iSCNT) technique
Biochem/physiol Actions
6-(Dimethylamino)purine (6-DMAP) is a protein kinase and cyclin-dependent kinase inhibitor. It acts as a secondary metabolite and mediates RNA modification. 6-DMAP is a potent cytokinetic inhibitor and is used in parthenogenesis and meiosis studies. It is also used to promote pronuclei formation in mammalian oocytes. 6-DMAP is a dual fluorescence molecule according to femtosecond fluorescence up-conversion spectroscopy studies.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kyung H Chang et al.
Fertility and sterility, 80(6), 1380-1387 (2003-12-12)
To establish an interspecies somatic cell nuclear transfer (iSCNT) technique for deriving blastocysts having human chromosome complements without sacrificing human oocytes. Prospective, randomized study undertaken in vitro. University-affiliated hospital and laboratory, Seoul National University. Postpartum women with natural spontaneous vaginal
FTIR and FT-Raman spectra of 6-(dimethylamino) purine and its theoretical studies of anharmonic vibrational analysis using quantum chemical calculations
Faizan M and Ahmad S
Vibrational Spectroscopy, 113, 103224-103224 (2021)
Y-p Hou et al.
Theriogenology, 72(5), 643-649 (2009-07-07)
The objective was to compare various activation protocols on developmental potential of vitrified bovine oocytes. Bovine oocytes matured in vitro for 23 h were vitrified with EDFSF30 in open pulled straws. After warming, they were cultured in vitro for 1h
Lizhi Leng et al.
Scientific reports, 7(1), 4242-4242 (2017-06-28)
A diploid genome is necessary for normal mammalian development, thus haploid parthenogenetic embryos undergo frequent self-diploidization during preimplantation development; however, the underlying mechanism is unclear. In this study, time-lapse recording revealed that human haploid parthenotes (HPs) undergo self-diploidization via failed
S Bhojwani et al.
Theriogenology, 67(2), 341-345 (2006-09-27)
The aim of the present investigation was to study the effect of oocyte selection on the efficiency of bovine nuclear transfer in terms of increased blastocyst production. For this purpose, prior to in vitro maturation (IVM), oocytes were selected for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service