Recommended Products
General description
The Oxyrase Enzyme System is obtained from the cytoplasmic membrane of E, coli
Application
EC-Oxyrase® has been used as a component of imaging buffer for stochastic optical reconstruction microscopy (STORM) imaging.
Oxyrase is an enzyme system that offers a way to remove dissolved oxygen from liquid, gas or semisolid products. Oxyrase utilizes the same mechanics of respiration employed by all living beings. It selectively captures oxygen and with a substrate converts it to water. Oxyrase is equivalent to a “strong” reducing agent, but unlike chemical antioxidants, Oxyrase, the substrates and reactants are all-natural. Oxyrase is designed to work efficiently over a wide range of pH and temperature
Various applications of Oxyrase include the preparation of anaerobic media for culture of anaerobic microorganisms, and the protection of oxygen-sensitive materials. In studies of biological processes, Oxyrase can help to enhance the intensity and longevity of activated fluorescent dye which are at risk of oxygen quenching.
Biochem/physiol Actions
EC-Oxyrase is active over a wide temperature range. It can be kept at 40 °C for hours without substantial loss of activity. It is heat inactivated above 55°C.
EC-Oxyrase operates over a wide pH range of 6.8 to 9.4. The optimum pH is about 8.0. As the pH moves away from optimum, activity decreases. The lower activity level can be compensated for by increasing time to complete oxygen removal or by increasing EC-Oxyrase concentration.
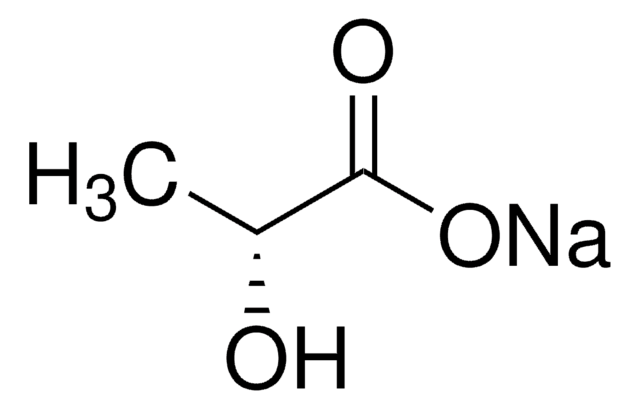
EC-Oxyrase requires substrate to be active. Levels at 10 mM to 20 mM are adequate. Substrates for EC Oxyrase include lactic acid, succinic acid, formic acid or their salts, and alpha-glycerol phosphate inaddition to oxygen. EC Oxyrase also contains L-sodium lactate at 100 mM to stabilize the Oxyrase during freeze-thaw cycles. This amount of substrate is diluted to extinction upon use.
The exact volume of EC Oxyrase and substrates needed to reduce oxygen in a given system are determined by a number of parameters: including pH, temperature, kinds and amounts of substrates present, surface to depth ratio of the container, and headspace volume. Some experimentation may be necessary; a suggested use level is a 1:100 dilution of the product.
The exact volume of EC Oxyrase and substrates needed to reduce oxygen in a given system are determined by a number of parameters: including pH, temperature, kinds and amounts of substrates present, surface to depth ratio of the container, and headspace volume. Some experimentation may be necessary; a suggested use level is a 1:100 dilution of the product.
Analysis Note
EC Oxyrase contains a penicillin binding protein that may interfere with penicillin and some related antibiotics.
Legal Information
Oxyrase is a registered trademark of Oxyrase, Inc.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jiegen Wu et al.
Molecular systems biology, 16(5), e9335-e9335 (2020-05-15)
Recent studies have revealed that global extrinsic noise arising from stochasticity in the intracellular biochemical environment plays a critical role in heterogeneous cell physiologies. However, it remains largely unclear how such extrinsic noise dynamically influences downstream reactions and whether it
Super-resolution imaging of synaptic and Extra-synaptic AMPA receptors with different-sized fluorescent probes.
Lee S H, et al.
eLife, 6, e27744-e27744 (2017)
Edwin Garcia et al.
The journal of pathology. Clinical research, 7(5), 438-445 (2021-05-22)
Electron microscopy (EM) following immunofluorescence (IF) imaging is a vital tool for the diagnosis of human glomerular diseases, but the implementation of EM is limited to specialised institutions and it is not available in many countries. Recent progress in fluorescence
Jasmer Dalal et al.
Molecular reproduction and development, 87(10), 1048-1058 (2020-08-12)
The objective of this study was to determine the effectiveness of deoxygenation of semen extender using Escherichia coli membrane-derived oxygen scavenger (Oxyrase) on post-thaw quality of buffalo (Bubalus bubalis) spermatozoa. Sixteen semen ejaculates, four each from four bulls, were each
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service