124168
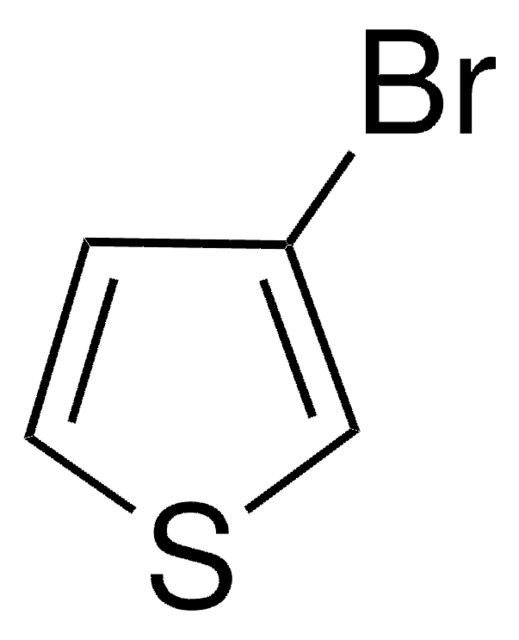
2-Bromothiophene
98%
Synonym(s):
2-Thienyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H3BrS
CAS Number:
Molecular Weight:
163.04
Beilstein:
104663
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.586 (lit.)
bp
149-151 °C (lit.)
density
1.684 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
Brc1cccs1
InChI
1S/C4H3BrS/c5-4-2-1-3-6-4/h1-3H
InChI key
TUCRZHGAIRVWTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Bromothiophene undergoes metalation-alkylation reaction with various electrophiles to form various 5-alkylated 2-bromo products. It undergoes coupling with 4-bromo allyl phenyl ether to form allyl phenyl thiophene ether, which is a novel potential dielectric material for organic thin film transistors.
Application
2-Bromothiophene has been used in electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium perchlorate by cyclic voltammetry and controlled-potential electrolysis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Eye Dam. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel self assembled monolayers of allyl phenyl thiophene ether as potential dielectric material for organic thin film transistors.
Sathyapalan A, et al
Thin Solid Films, 516(16), 5645-5648 (2008)
R A Ingle et al.
The Journal of chemical physics, 147(1), 013914-013914 (2017-07-10)
The ultraviolet photochemistry of 2-bromothiophene (C
Synlett, 974-974 (2007)
First example of base-promoted tandem alkylation-bromination of 2-bromothiophene via halogen dance process: a remarkable temperature effect.
Peyron C, et al.
Tetrahedron Letters, 46(19), 3315-3318 (2005)
Mohammad S. Mubarak et al.
The Journal of organic chemistry, 61(23), 8074-8078 (1996-11-15)
Cyclic voltammetry and controlled-potential electrolysis have been employed to probe the electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium perchlorate. Reduction of 2-bromo-, 3-bromo-, 2-chloro-, 3-chloro-, and 2-iodothiophene gives rise to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service