164283
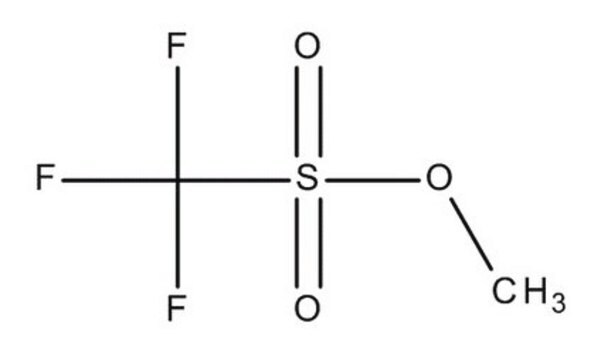
Methyl trifluoromethanesulfonate
≥98%
Synonym(s):
Methyl triflate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3SO2OCH3
CAS Number:
Molecular Weight:
164.10
Beilstein:
774772
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.326 (lit.)
bp
94-99 °C (lit.)
density
1.45 g/mL at 25 °C (lit.)
functional group
fluoro
triflate
storage temp.
2-8°C
SMILES string
COS(=O)(=O)C(F)(F)F
InChI
1S/C2H3F3O3S/c1-8-9(6,7)2(3,4)5/h1H3
InChI key
OIRDBPQYVWXNSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl trifluoromethanesulfonate is a strong methylating reagent, commonly used for the pre-methylation of polysaccharides under mild basic conditions.
Application
Methyl trifluoromethanesulfonate can be used as a methylation reagent:
- In the determination of polysulfides, zerovalent sulfur in sulfide-rich water wells, and polysulfide species in electrolyte of a lithium–sulfur battery using chromatography-based techniques.
- In reactions with potassium enolates.
- For the conversion of amines to methyl ammonium triflates.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
100.4 °F - closed cup
Flash Point(C)
38 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Quantitative and Qualitative Determination of Polysulfide Species in the Electrolyte of a Lithium?Sulfur Battery using HPLC ESI/MS with One?Step Derivatization
Zheng D, et al.
Advanced Energy Materials, 5(16) (2015)
Speciation of polysulfides and zerovalent sulfur in sulfide-rich water wells in southern and central Israel
Kamyshny A, et al.
Aquatic Geochemistry, 14(2), 171-192 (2008)
Analytical Methods in Wood Chemistry, Pulping, and Papermaking (1998)
Purification of Laboratory Chemicals, 6 (2009)
Ring Expansions of 2-Alkenylazetidinium Salts-a New Route to Pyrrolidines and Azepanes
Couty F, et al.
European Journal of Organic Chemistry, 2006(18), 4214-4223 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service