All Photos(1)
About This Item
Empirical Formula (Hill Notation):
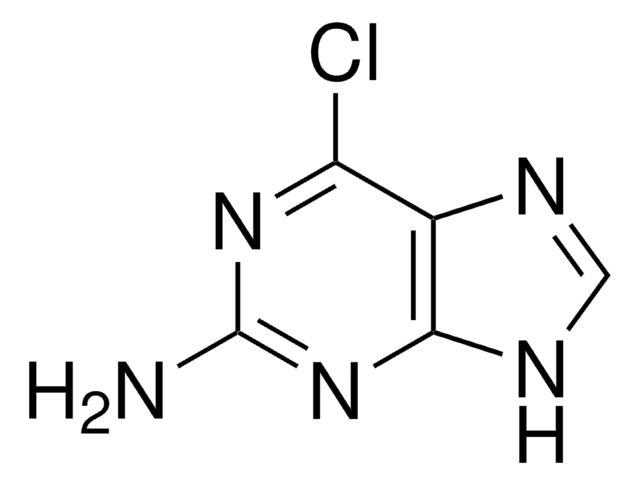
C5H6N6
CAS Number:
Molecular Weight:
150.14
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
mp
>300 °C (lit.)
SMILES string
Nc1nc(N)c2nc[nH]c2n1
InChI
1S/C5H6N6/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H5,6,7,8,9,10,11)
InChI key
MSSXOMSJDRHRMC-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Makoto Komiyama et al.
Nature protocols, 3(4), 646-654 (2008-04-05)
Pseudo-complementary peptide nucleic acid (pcPNA) is a DNA analog in which modified DNA bases 2,6-diaminopurine (D) and 2-thiouracil (U(s)) 'decorate' a poly[N-(2-aminoethyl)glycine] backbone, together with guanine (G) and cytosine (C). One of the most significant characteristics of pcPNA is its
Anna K Shchyolkina et al.
Nucleic acids research, 34(11), 3239-3245 (2006-06-27)
Several cellular processes involve alignment of three nucleic acids strands, in which the third strand (DNA or RNA) is identical and in a parallel orientation to one of the DNA duplex strands. Earlier, using 2-aminopurine as a fluorescent reporter base
Li Qiu et al.
PLoS neglected tropical diseases, 12(4), e0006421-e0006421 (2018-04-20)
Dengue virus affects millions of people worldwide each year. To date, there is no drug for the treatment of dengue-associated disease. Nucleosides are effective antivirals and work by inhibiting the accurate replication of the viral genome. Nucleobases offer a cheaper
Miguel A Galindo et al.
Inorganic chemistry, 48(23), 11085-11091 (2009-10-28)
Alkyldiamine-tethered derivatives of 2,6-diaminopurine, ethylenediamine-N9-propyl-2,6-diaminopurine, L1, and ethylenediamine-N9-ethyl-2,6-diaminopurine, L2, react with Pd(II) to give N3-coordinated complexes. However, the exact nature of the resulting complex is dependent on the reaction conditions. With PdCl(2)(MeCN)(2) in MeCN/H(2)O the expected [PdCl(N3-2,6-DAP-alkyl-en)](+) complex, 1, is
Søren Lindemose et al.
Nucleic acids research, 36(14), 4797-4807 (2008-07-26)
The DNA interaction of the Escherichia coli cyclic AMP receptor protein (CRP) represents a typical example of a dual recognition mechanism exhibiting both direct and indirect readout. We have dissected the direct and indirect components of DNA recognition by CRP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service