252727
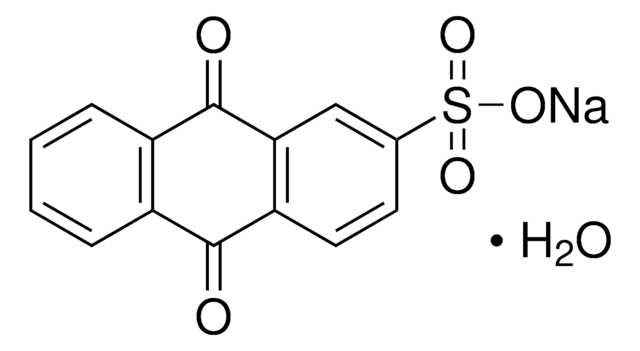
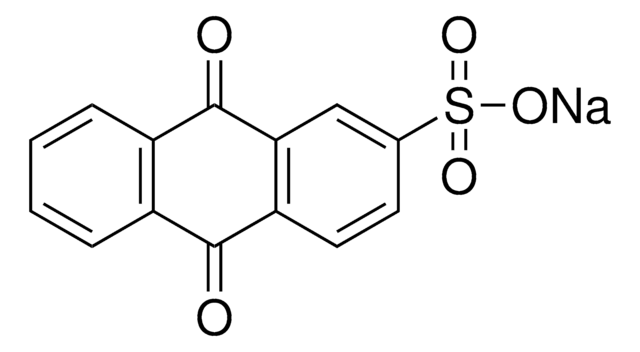
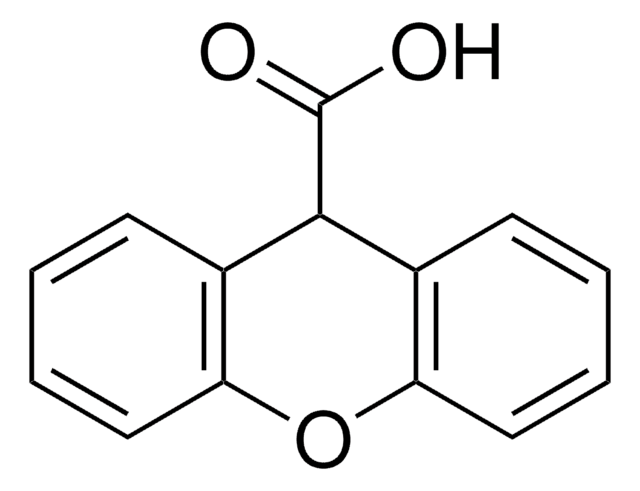
Anthraquinone-2-carboxylic acid
98%
Synonym(s):
9,10-Dihydro-9,10-dioxo-2-anthracenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H8O4
CAS Number:
Molecular Weight:
252.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
287-289 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
OC(=O)c1ccc2C(=O)c3ccccc3C(=O)c2c1
InChI
1S/C15H8O4/c16-13-9-3-1-2-4-10(9)14(17)12-7-8(15(18)19)5-6-11(12)13/h1-7H,(H,18,19)
InChI key
ASDLSKCKYGVMAI-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H H Cheng et al.
Research communications in molecular pathology and pharmacology, 105(1-2), 97-103 (2000-06-13)
The objectives of this study are to describe the inhibitory effect of 9,10-anthraquinone 2-carboxylic acid (AQCA) on IgE-mediated passive cutaneous anaphylaxis (PCA) reaction, and the pharmacokinetics of AQCA. Pharmacodynamic assessments were performed at 0.5, 1 and 2 mg/kg (i.v.) and
Chunyuan Wu et al.
Frontiers in microbiology, 11, 2003-2003 (2020-09-29)
Due to toxicity and persistence of paraquat (a widely used herbicide), eco-friendly remediation approaches to its contamination and effective antidotes to its poisoning have been highly desired and raised increasing concerns. Paraquat degradation was lesser in aerobic soil in comparison
A Bielawska et al.
Folia histochemica et cytobiologica, 39 Suppl 2, 207-208 (2002-02-01)
Although prolidase [E.C.3.4.13.9] is found in normal cells, substantially increased levels are found in some neoplastic tissues. Prolidase evokes the ability to hydrolyse the imido-bond of various low molecular weight compounds coupled to L-proline. The synthesis of three proline analogues
A Bielawska et al.
Roczniki Akademii Medycznej w Bialymstoku (1995), 43, 201-209 (1999-02-11)
The feasibility to targeting prolidase as an antineoplastic prodrug--converting enzyme has been examined. The synthesis of proline analogue of anthraquinone-2-carboxylic acid (potential antineoplastic agent) conjugated through imido-bond (potential target for prolidase action) has been performed. The product was found to
A Bielawska et al.
Polish journal of pharmacology, 53(3), 283-287 (2002-01-12)
A series of proline analogues of anthraquinone-2-carboxylic acid (1-3) were synthesized and evaluated for cytotoxic activity in the cultured breast cancer MCF-7 cells. The concentrations of 1, 2 and 3 needed to inhibit [3H]thymidine incorporation into DNA by 50% (IC50)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service