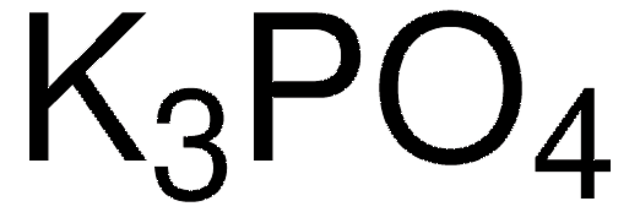All Photos(1)
About This Item
Linear Formula:
NCCH2CSNH2
CAS Number:
Molecular Weight:
100.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
118-120 °C (lit.)
functional group
amine
nitrile
SMILES string
NC(=S)CC#N
InChI
1S/C3H4N2S/c4-2-1-3(5)6/h1H2,(H2,5,6)
InChI key
BHPYMZQTCPRLNR-UHFFFAOYSA-N
Related Categories
Application
2-Cyanothioacetamide was used in the synthesis of 3,4-trans-4-aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates and bis[6-(2-aryl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile]. It was also used as a building block for 2-pyridothiones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liebigs Ann. Chem., 213-213 (1984)
Liebigs Ann. Chem., 210-210 (1986)
Farag M A Altalbawy
International journal of molecular sciences, 14(2), 2967-2979 (2013-02-01)
The title compounds were prepared by reaction of 1,1'-(5-methyl-1-phenyl-1H-pyrazole-3,4-diyl)diethanone (1) with different aromatic aldehydes 2a-c, namely Furfural (2a), 4-chlorobenzaldehyde (2b) and 4-methoxybenzaldhyde (2c) to yield the corresponding α,β-unsaturated ketones 3a-c. Compound 3 was reacted with malononitrile, 2-cyanoacetamide or 2-cyanothioacetamide yielded
A Krauze et al.
European journal of medicinal chemistry, 40(11), 1163-1167 (2005-06-02)
3,4-trans-4-Aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates 6-11 were prepared by a Michael reaction of N-acetonylpyridinium chloride with 3-aryl-2-cyanothioacrylamides or by a one-pot three-carbon condensation of N-acetonylpyridinium chloride, aromatic aldehyde and 2-cyanothioacetamide, and their cardiotonic properties were studied. 3,4-trans-5-cyano-2-hydroxy-2-methyl-4-(3-nitrophenyl)-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolate 8 was considered as a lead compound
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service