All Photos(1)
About This Item
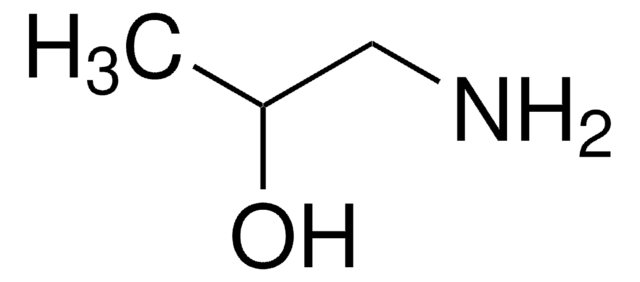
Linear Formula:
H2C=C(NHCOCH3)CO2CH3
CAS Number:
Molecular Weight:
143.14
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
bp
104 °C/8 mmHg (lit.)
mp
50-52 °C (lit.)
SMILES string
COC(=O)C(=C)NC(C)=O
InChI
1S/C6H9NO3/c1-4(6(9)10-3)7-5(2)8/h1H2,2-3H3,(H,7,8)
InChI key
SMWNFFKPVLVOQQ-UHFFFAOYSA-N
Related Categories
General description
Conjugated addition of secondary amines, imidazole and pyrazole to methyl 2 methyl 2-acetamidoacrylate in the presence of a catalyst results in the formation of β-Dialkylamino-α-alanine and β-(N-heteroaryl)-α-alanine derivatives. Methyl-2-acetamidoacrylate (M2AA) is an anti-inflammatory agent. The catalytic reaction of methyl 2-acetamidoacrylate with Grignard′s reagents affords α-amino esters. M2AA can form thermosensitive copolymers with methyl acrylate.
Methyl ester of 2-acetamidoacrylate . methyl 2-acetamidoacrylate (Me-2-AA) is a di-unsaturated α-amino acid derivative. methyl-2-acetamidoacrylate exihibits anti -inflammatory properties, it is very effective against lipopolysaccharide (LPS)- induced nitric oxide production by RAW 264.
Application
Methyl 2-acetamidoacrylate can undergo [2+2] cycloaddition (Michael–Dieckmann-type reaction) with ketene diethyl acetal to yield the cyclobutane core. It may be used in rhodium-catalyzed 2-alkenylpyrrole formation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
FeCl3-catalyzed conjugate addition of secondary amines, imidazole and pyrazole to methyl 2-acetamidoacrylate.
Montserrat P and Pleixats R
Tetrahedron, 51(30), 8355-8362 (1995)
Ethyl pyruvate: a novel anti?inflammatory agent.
Fink MP, et al.
J. Int. Med., 261(4), 349-362 (2007)
Madeleine E Kieffer et al.
Journal of the American Chemical Society, 134(11), 5131-5137 (2012-03-07)
The tandem Friedel-Crafts conjugate addition/asymmetric protonation reaction between 2-substituted indoles and methyl 2-acetamidoacrylate is reported. The reaction is catalyzed by (R)-3,3'-dibromo-BINOL in the presence of stoichiometric SnCl(4), and is the first example of a tandem conjugate addition/asymmetric protonation reaction using
Thermosensitive properties of a novel poly(methyl 2-acetamidoacrylate-co-methyl acrylate)
Okamura H, et al.
European Polymer Journal, 38(4), 639-644 (2002)
Benoît Cossec et al.
Molecules (Basel, Switzerland), 13(10), 2394-2407 (2008-10-03)
A safe and simple method for methyl S-arylmercapturate synthesis is described. Thirteen such compounds, to be used afterwards in metabolism studies, have been obtained with yields ranging from 71 to 99.6%. These compounds were obtained using a sulfa-Michael addition and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service