All Photos(1)
About This Item
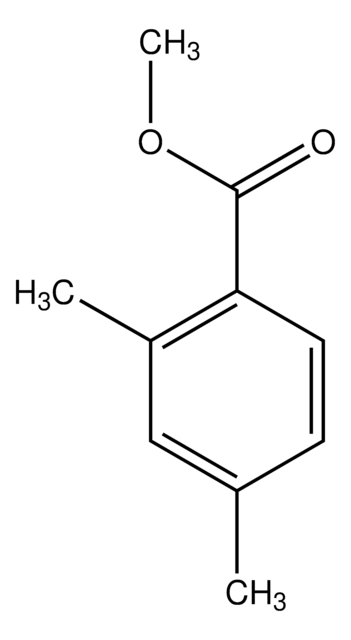
Linear Formula:
(CH3)2C6H3CO2CH3
CAS Number:
Molecular Weight:
164.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
239-240 °C (lit.)
mp
31-33 °C (lit.)
density
1.027 g/mL at 25 °C (lit.)
SMILES string
COC(=O)c1cc(C)cc(C)c1
InChI
1S/C10H12O2/c1-7-4-8(2)6-9(5-7)10(11)12-3/h4-6H,1-3H3
InChI key
PEVXENGLERTHJE-UHFFFAOYSA-N
Related Categories
General description
Methyl 3,5-dimethylbenzoate is an aromatic carboxylic acid ester. It was selected as ligand during monomer screening for the synthesis and investigation of various europium compounds containing pinacolyl methylphosphonate with different ligands. Methyl 3,5-dimethylbenzoate is reported as precursor of methyl-3,5-divinylbenzoate.
Application
Methyl 3,5-dimethylbenzoate may be used in the preparation of four carbon isostere related to highly active 4-pyridinemethanols, which were subsequently evaluated for their antimalarial activity. It may be used in the total synthesis of (±)-indoxamycin B.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oliver F Jeker et al.
Angewandte Chemie (International ed. in English), 51(14), 3474-3477 (2012-02-22)
Revised version: the first total synthesis of indoxamycin B leads to a stereochemical reassignment of the natural product. The synthetic route features an efficient carboannulation sequence to rapidly access the dihydroindenone system. Moreover, a series of Au(I)-catalyzed transformations served in
Molecularly imprinted polymers for the selective sequestering and sensing of ions.
The Johns Hopkins Medical Letter Health After 50, 18(4), 465-465 (1997)
A Markovac et al.
Journal of medicinal chemistry, 23(11), 1198-1201 (1980-11-01)
Four carbon isosteres related to the highly active 4-pyridylcarbinolamines were prepared and evaluated for suppressive antimalarial activity against Plasmodium berghei in mice. Three of the four examples possessed significant activity but were approximately one dose level less active than the
A L Jenkins et al.
Analytical chemistry, 71(2), 373-378 (1999-02-09)
The techniques of molecular imprinting and sensitized lanthanide luminescence have been combined to create the basis for a sensor that can selectively measure the hydrolysis product of the nerve agent Soman in water. The sensor functions by selectively and reversibly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service