All Photos(2)
About This Item
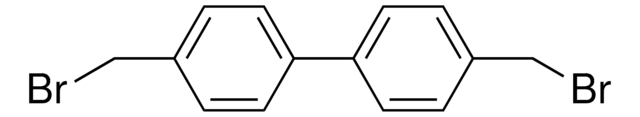
Linear Formula:
ClCH2C6H4C6H4CH2Cl
CAS Number:
Molecular Weight:
251.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
126 °C (dec.) (lit.)
functional group
chloro
SMILES string
ClCc1ccc(cc1)-c2ccc(CCl)cc2
InChI
1S/C14H12Cl2/c15-9-11-1-5-13(6-2-11)14-7-3-12(10-16)4-8-14/h1-8H,9-10H2
InChI key
INZDTEICWPZYJM-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chang-Tao Hsiao et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(4), 1358-1364 (2011-01-19)
Pyrolytic carbon thin films were deposited on quartz plates through a chemical vapor deposition process, by using a biphenyl precursor, 4,4'-bis(chloromethyl-1,1'-biphenyl). The pyrolytic carbons were microporous and catalytic toward reduction of tri-iodide, and the films thus obtained possessed a metallic
Reaction of 4, 4'-bis (chloromethyl)-1, 1'-biphenyl and phenol in two-phase medium via phase-transfer catalysis.
Wang M-L and Lee Z-F.
J. Mol. Catal. A: Chem., 264(1), 119-127 (2007)
Lukas Rübenach et al.
ChemSusChem, 12(15), 3627-3634 (2019-05-10)
The utilization of biomass is one of the major challenges for the transition from fossil to renewable resources. Often, the separation of the desired product from the reaction mixture is the most energy-intensive step. Liquid-phase adsorption is a promising separation
Hung-Ming Yang et al.
Ultrasonics sonochemistry, 21(1), 395-400 (2013-08-27)
The catalytic esterification of sodium 4-hydroxybenzoate with benzyl bromide by ultrasound-assisted solid-liquid phase-transfer catalysis (U-SLPTC) was investigated using the novel dual-site phase-transfer catalyst 4,4'-bis(tributylammoniomethyl)-1,1'-biphenyl dichloride (BTBAMBC), which was synthesized from the reaction of 4,4'-bis(chloromethyl)-1,1'-biphenyl and tributylamine. Without catalyst and in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service