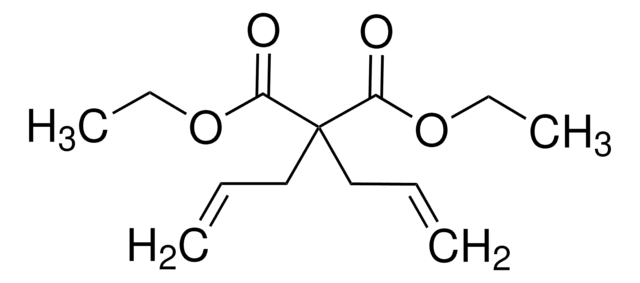
569747
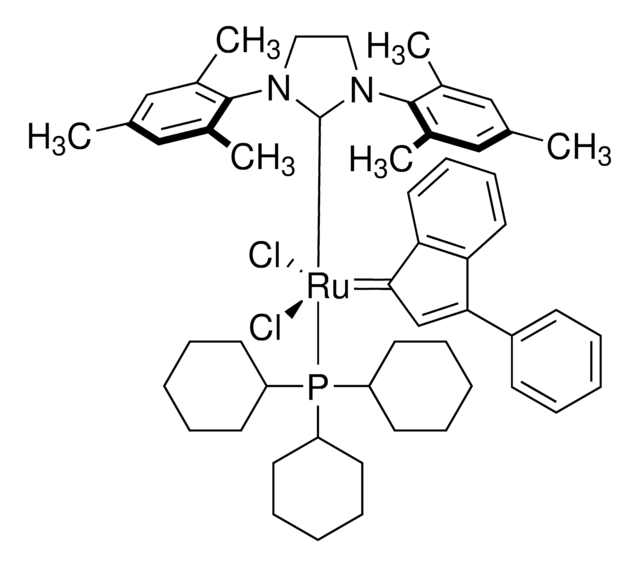
Grubbs Catalyst® M204
Umicore
Synonym(s):
(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium, Benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(tricyclohexylphosphine)ruthenium, Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](benzylidene)(tricyclohexylphosphine)ruthenium(II), Grubbs Catalyst® 2nd Generation, Grubbs Catalyst® M2a (C848)
About This Item
Recommended Products
Quality Level
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
mp
143.5-148.5 °C
storage temp.
2-8°C
SMILES string
CC1=CC(C)=CC(C)=C1N2CCN(C3=C(C)C=C(C)C=C3C)C2=[Ru](Cl)(Cl)=CC4=CC=CC=C4.P(C5CCCCC5)(C6CCCCC6)C7CCCCC7
InChI
1S/C21H26N2.C18H33P.C7H6.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1-6H;2*1H;/q;;;;;+2/p-2
InChI key
FCDPQMAOJARMTG-UHFFFAOYSA-L
Application
It can also be used as a catalyst:
- To synthesize coumarins from phenolic compounds via RCM.
- To cleave secondary (E)-allyl vic-diols to aldehydes.
Learn more about our metathesis catalysts
Other Notes
Legal Information
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn tips and tricks for how to properly use inhibitors including how to select the right inhibitor and how to plan experiments with inhibitors.
Protocols
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
Related Content
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service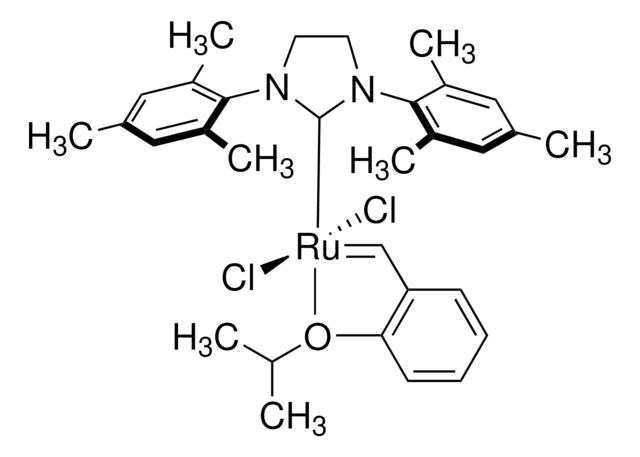
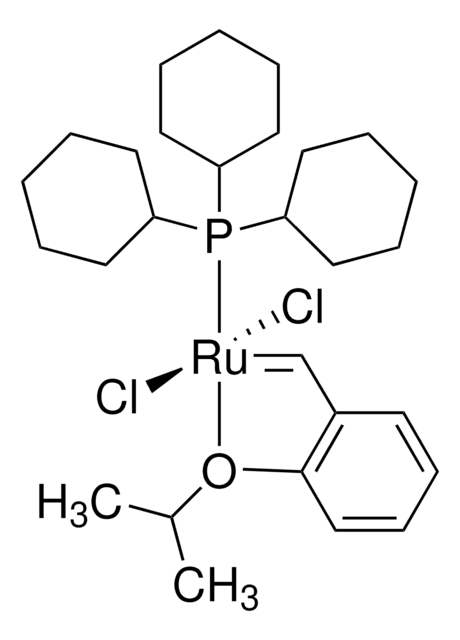


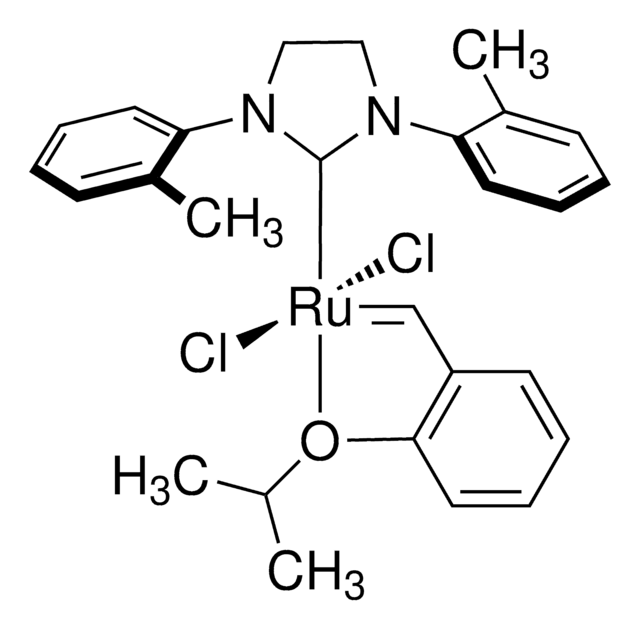
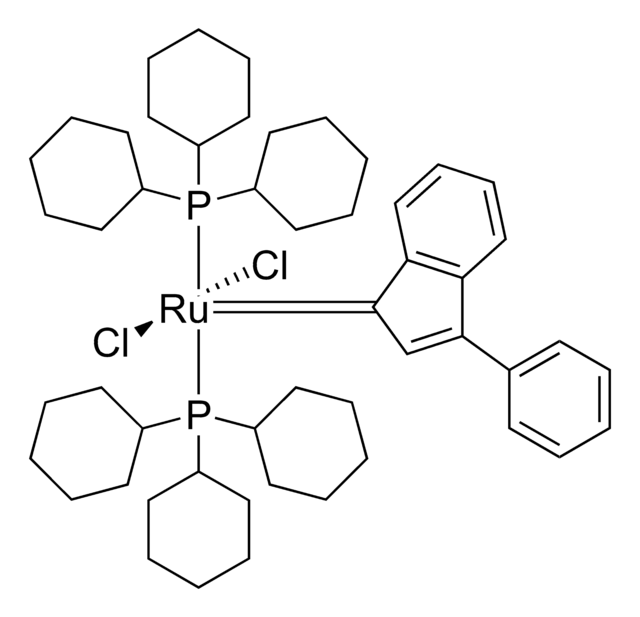
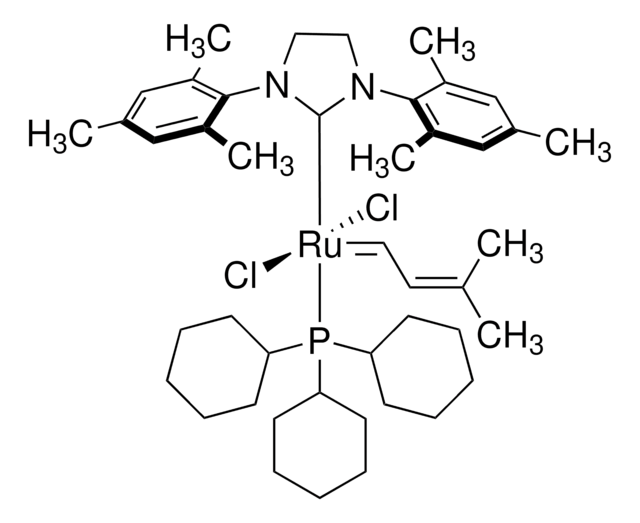

![2,6-Diisopropylphenylimido-neophylidene[(S)-(−)-BIPHEN]molybdenum(VI) ringclosing metathesis catalyst, ≥95.0% (C)](/deepweb/assets/sigmaaldrich/product/structures/312/745/96ea840b-77a7-427a-9db5-fa08b3ffd45e/640/96ea840b-77a7-427a-9db5-fa08b3ffd45e.png)
![Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene][[5-[(dimethylamino)sulfonyl]-2-(1-methylethoxy-O)phenyl]methylene-C]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/179/573/f48a2a1e-cf09-4151-8b78-2bab614efd5c/640/f48a2a1e-cf09-4151-8b78-2bab614efd5c.png)