638072
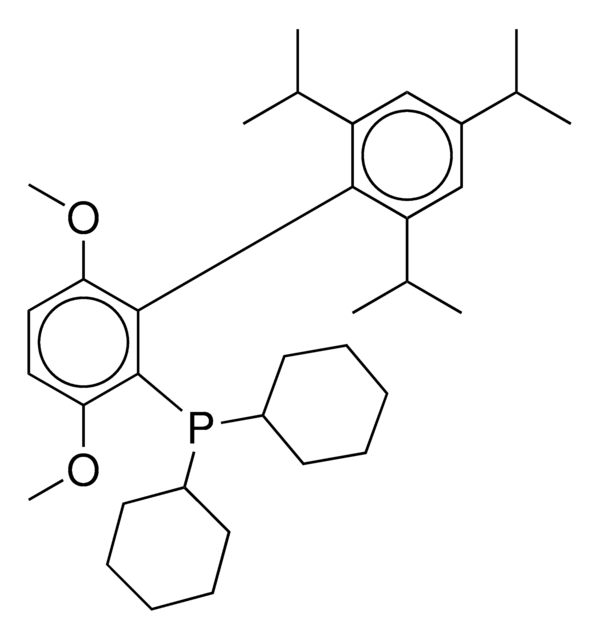
SPhos
98%
Synonym(s):
SPhos, 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl, S Phos
About This Item
Recommended Products
Quality Level
Assay
98%
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Kumada Coupling
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
greener alternative product score
old score: 10
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Less Hazardous Chemical Syntheses
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
164-166 °C (lit.)
functional group
phosphine
greener alternative category
SMILES string
COc1cccc(OC)c1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C26H35O2P/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21/h9-11,16-21H,3-8,12-15H2,1-2H3
InChI key
VNFWTIYUKDMAOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
For small scale and high throughput uses, product is also available as ChemBeads (932191)
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between Boc-protected aminomethyltrifluoroborate and aryl chlorides or hetaryl chlorides to form the corresponding aminomethylarenes.
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between 4-methyl-substituted piperidinylzinc reagent and different aryl or heteroaryl iodides to form various substituted piperidines.
- Intramolecular Suzuki-Miyaura coupling to form the 18-membered macrocyclic ring during the multi-step synthesis of riccardin C.
Legal Information
related product
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald Phosphine Ligands
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Related Content
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)