A77903
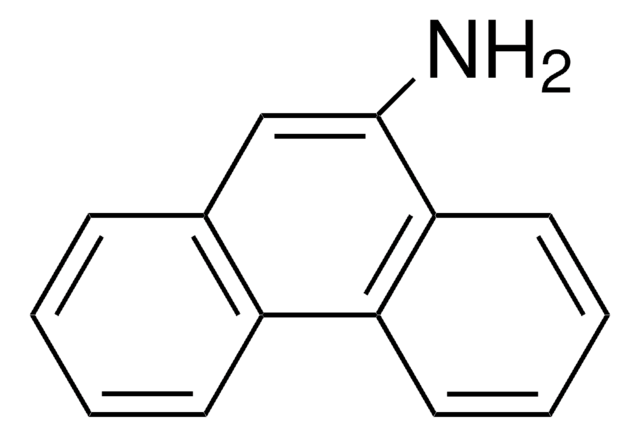
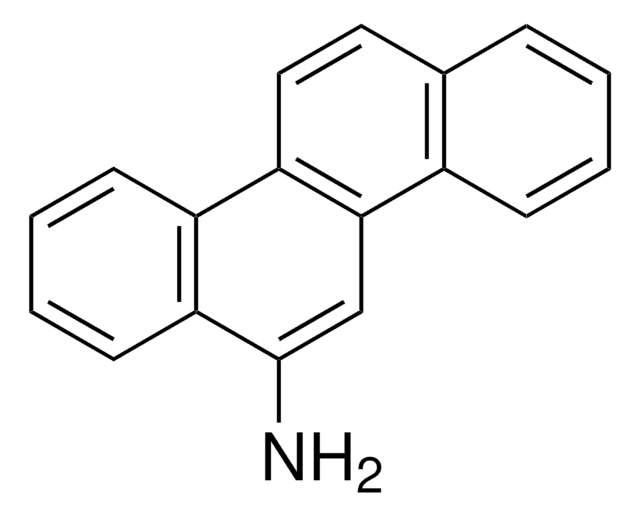
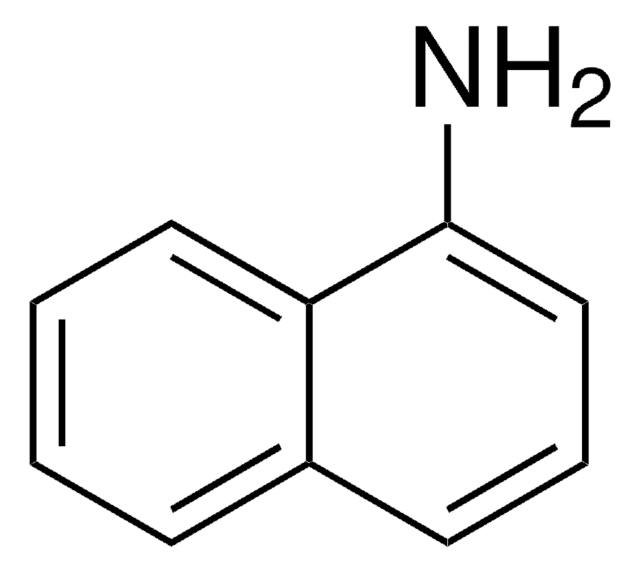
1-Aminopyrene
97%
Synonym(s):
1-Pyrenamine, 3-Aminopyrene
About This Item
Recommended Products
Assay
97%
form
powder
mp
115-117 °C (lit.)
SMILES string
Nc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C16H11N/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9H,17H2
InChI key
YZVWKHVRBDQPMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Synthesis and evaluation of aromatic stationary phases based on linear solvation energy relationship model for expanded application in supercritical fluid chromatography.: Explores the use of 1-Aminopyrene in the development of advanced stationary phases for chromatography, enhancing the efficiency and application range in pharmaceutical and environmental analysis (Ge et al., 2024).
- Two-Dimensional Porphyrinic Metal-Organic Framework Composites as a Photocatalytic Platform for Chemoselective Hydrogenation.: Highlights the integration of 1-Aminopyrene into metal-organic frameworks, providing a new avenue for creating highly efficient photocatalytic systems for organic synthesis, relevant to both academic research and industrial chemical manufacturing (Dong et al., 2023).
- Exploiting and Engineering Neuroglobin for Catalyzing Carbene N-H Insertions and the Formation of Quinoxalinones.: Discusses the role of 1-Aminopyrene in enhancing enzymatic reactions within engineered proteins, paving the way for novel synthetic pathways in pharmaceutical research (Sun et al., 2023).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service