C112208
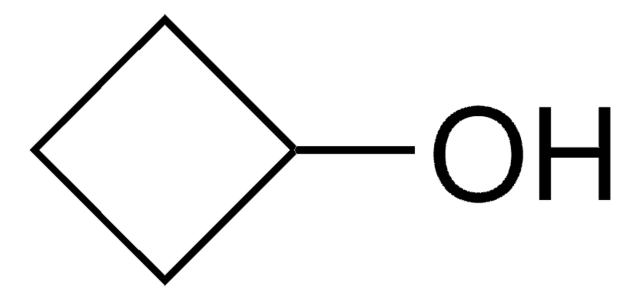
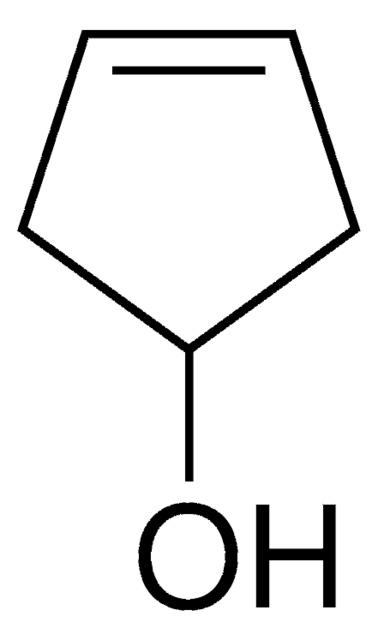
Cyclopentanol
99%
Synonym(s):
1-Cyclopentanol, Cyclopentyl alcohol, Hydroxycyclopentane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C5H9OH
CAS Number:
Molecular Weight:
86.13
Beilstein:
1900556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.453 (lit.)
bp
139-140 °C (lit.)
mp
−19 °C (lit.)
density
0.948 g/mL at 20 °C
0.949 g/mL at 25 °C (lit.)
SMILES string
OC1CCCC1
InChI
1S/C5H10O/c6-5-3-1-2-4-5/h5-6H,1-4H2
InChI key
XCIXKGXIYUWCLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclopentanol can be used as:
- An alkylating agent in the preparation of alkylated aromatic compounds using Fe3+-montmorillonite catalyst via Friedel–Crafts alkylation reaction.
- A reactant in the acylation of alcohols with an acid anhydride or acid chloride.
- A substrate in the synthesis of high-density polycyclic aviation fuel by the Guerbet reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
123.8 °F - closed cup
Flash Point(C)
51 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daisuke Yamashita et al.
Angewandte Chemie (International ed. in English), 48(8), 1404-1406 (2008-12-05)
No bones about it: (-)-Norzoanthamine, a promising candidate for an anti-osteoporotic drug, was the target of a total synthesis (see scheme). The final bisaminal formation with AcOH/H(2)O gave the DEFG ring, while the cyclization precursor was prepared by installing the
Fatty acid signalling in plants and their associated microorganisms.
E E Farmer
Plant molecular biology, 26(5), 1423-1437 (1994-12-01)
Hiroaki Iwaki et al.
Applied and environmental microbiology, 68(11), 5671-5684 (2002-10-31)
Cyclopentanone 1,2-monooxygenase, a flavoprotein produced by Pseudomonas sp. strain NCIMB 9872 upon induction by cyclopentanol or cyclopentanone (M. Griffin and P. W. Trudgill, Biochem. J. 129:595-603, 1972), has been utilized as a biocatalyst in Baeyer-Villiger oxidations. To further explore this
José Barluenga et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(27), 7225-7235 (2006-08-24)
The one-pot sequential reaction of a chromium alkoxycarbene complex, a ketone or ester lithium enolate, and allylmagnesium bromide enabled the selective synthesis of novel diastereomerically pure pentasubstituted cyclopentanols or tetrasubstituted 1,4-cyclohexanediols, depending on the degree of substitution at the Cbeta
Marie Bøjstrup et al.
Organic & biomolecular chemistry, 3(9), 1738-1745 (2005-04-29)
Four aminocyclopentanols, as mimics of putative intermediates in the hydrolysis of alpha-d-galactosides, have been synthesized through a number of stereoselective transformations using the cis-fused cyclopentane-1,4-lactone (1R, 5S, 7R, 8R)-7,8-dihydroxy-2-oxabicyclo[3.3.0]oct-3-one as a chiral building block. The compounds were tested towards various
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service