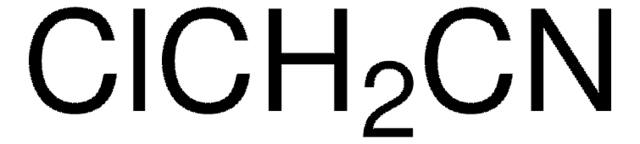C19651
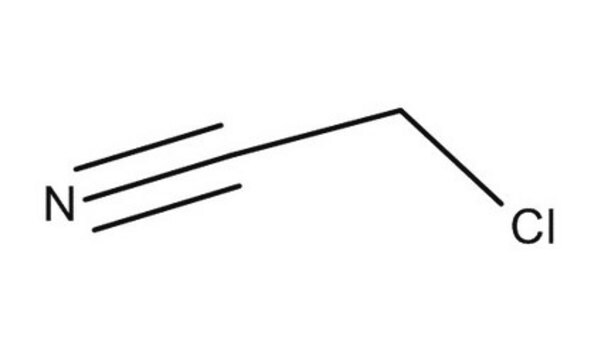
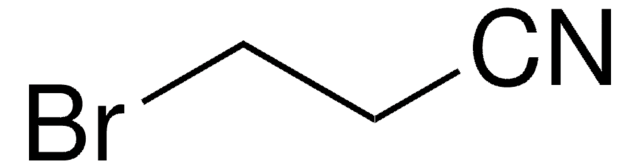
Chloroacetonitrile
99%
Synonym(s):
α-Chloroacetonitrile, 2-Chloroacetonitrile, Chloromethyl cyanide, Cyanomethyl chloride, Monochloroacetonitrile
About This Item
Recommended Products
vapor density
3 (vs air)
Quality Level
vapor pressure
1.78 psi ( 20 °C)
Assay
99%
form
liquid
refractive index
n20/D 1.422 (lit.)
bp
124-126 °C (lit.)
density
1.193 g/mL at 25 °C (lit.)
SMILES string
ClCC#N
InChI
1S/C2H2ClN/c3-1-2-4/h1H2
InChI key
RENMDAKOXSCIGH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- For the synthesis of ionic liquids containing nitrile functionalized imidazolium salts.
- For the synthesis of cyanomethyl dodecyl trithiocarbonate, a reversible addition-fragmentation chain transfer (RAFT) agent.
- As an alkylating reagent for the synthesis of derivatives of triazolyl thiazoles to be used as anti-tuberculosis agents.
- As a reagent in photo Meerwein addition reaction for amino-arylation of alkenes.
- As an esterifying agent in the synthesis of a cytotoxic natural product: apicularen A.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F
Flash Point(C)
54 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service