C409
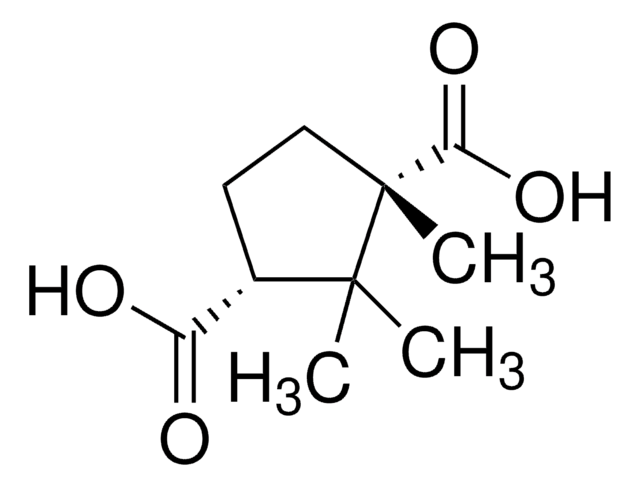
(1R,3S)-(+)-Camphoric acid
99%
Synonym(s):
(+)-Camphoric acid, (1R,3S)-1,2,2-Trimethyl-1,3-cyclopentanedicarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H16O4
CAS Number:
Molecular Weight:
200.23
Beilstein:
2050204
EC Number:
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical activity
[α]20/D 46°, c = 1 in ethanol
mp
183-186 °C (lit.)
SMILES string
CC1(C)[C@H](CC[C@@]1(C)C(O)=O)C(O)=O
InChI
1S/C10H16O4/c1-9(2)6(7(11)12)4-5-10(9,3)8(13)14/h6H,4-5H2,1-3H3,(H,11,12)(H,13,14)/t6-,10+/m1/s1
InChI key
LSPHULWDVZXLIL-LDWIPMOCSA-N
General description
Camphoric acid is a diacid, generally prepared by the oxidation of terpene (+)-camphor. It can be used as a chirality inducing agent in some organic reactions.
Application
(1R,3S)-(+)-Camphoric acid may be used in the preparation (1R,2S,3R,5S)-2,3-dibenzyl-1,8,8-trimethyl-3-thianiumbicyclo[3.2.1]octane perchlorate. It reacts with uranyl nitrate in pyridine(py) or py/methanol(MeOH) to form novel uranyl-organic assemblages.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solvothermal Synthesis and Crystal Structure of Uranyl Complexes with 1, 1-Cyclobutanedicarboxylic and (1R, 3S)-(+)-Camphoric Acids-Novel Chiral Uranyl-Organic Frameworks.
Thuery P.
European Journal of Inorganic Chemistry, 2006(18), 3646-3651 (2006)
Spontaneous resolution to absolute chiral induction: pseudo-kagome type homochiral Zn (II)/Co (II) coordination polymers with achiral precursors
Bisht KK and Suresh E
Journal of the American Chemical Society, 135(42), 15690-15693 (2013)
Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid
Nsengiyumva O and Miller SA
Green Chemistry, 21(5), 973-978 (2019)
Preparation of optically active epoxides via sulfur ylides. Origin of the chiral induction.
Breau L and Durst T.
Tetrahedron Asymmetry, 2(5), 367-370 (1991)
Carine Robert et al.
Nature communications, 2, 586-586 (2011-12-14)
The vast majority of commodity materials are obtained from petrochemical feedstocks. These resources will plausibly be depleted within the next 100 years, and the peak in global oil production is estimated to occur within the next few decades. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service