H11904
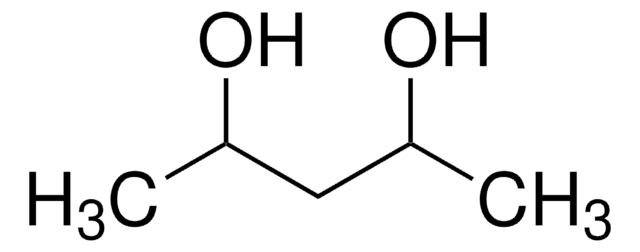
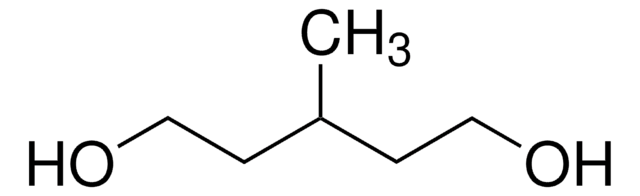
2,5-Hexanediol
99% (mixture of isomers)
Synonym(s):
2,5-Hexylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH2CH2CH(OH)CH3
CAS Number:
Molecular Weight:
118.17
Beilstein:
1719248
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99% (mixture of isomers)
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
216-218 °C (lit.)
density
0.961 g/mL at 25 °C (lit.)
SMILES string
CC(O)CCC(C)O
InChI
1S/C6H14O2/c1-5(7)3-4-6(2)8/h5-8H,3-4H2,1-2H3
InChI key
OHMBHFSEKCCCBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,5-Hexanediol can be used as:
- A reactant to synthesize N-substituted pyrroles by oxidative coupling with primary amines.
- An alkylating agent to synthesize substituted carbazoles via Friedel-Crafts alkylation with indoles.
- A monomer in the preparation of polyesters by reacting with diacid chlorides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Corey Frazer et al.
Nature microbiology, 5(11), 1374-1389 (2020-07-29)
Cell identity in eukaryotes is controlled by transcriptional regulatory networks that define cell-type-specific gene expression. In the opportunistic fungal pathogen Candida albicans, transcriptional regulatory networks regulate epigenetic switching between two alternative cell states, 'white' and 'opaque', that exhibit distinct host
T Yasui et al.
Sangyo eiseigaku zasshi = Journal of occupational health, 37(1), 19-24 (1995-01-01)
Rats were injected subcutaneously with 2,5-hexanedione (2,5-HD 2.6 m mol/kg) alone (HD group) or with 2,5-HD and methyl ethyl ketone (MEK) (2.6 m mol/kg of each agent, HD&MEK group) or with 2,5-HD 2.6 m mol/kg and 5 times that dose
H B Jones et al.
Acta neuropathologica, 58(4), 286-290 (1982-01-01)
Rats were given 2,5-hexanediol, a metabolite of n-hexane, in the drinking water until they developed a marked degree of paresis over about 7 weeks and were then allowed to recover naturally. The time course and the manner of removal of
J Haberland et al.
Applied microbiology and biotechnology, 58(5), 595-599 (2002-04-17)
Diastereoselective reduction of diketones with Lactobacillus kefir DSM 20587 was examined. The reduction of both oxo-functions proceeded highly diastereoselectively. (2 R,5 R)-Hexanediol 3 was produced starting from (2,5)-hexanedione 1 in quantitative yields with enantiomeric excess >99% and diastereomeric excess >99%.
K Kannan et al.
Environmental research, 36(1), 14-25 (1985-02-01)
A preliminary study on immunotoxicologic evaluation of 2,5-hexanediol (one of the principal metabolites of n-hexane), involving multiple immunological parameters, was carried out in mice. Mice were exposed to 2,5-hexanediol at a 1/5 LD50 dose level for 7 days. Pathotoxicological changes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service