M89617
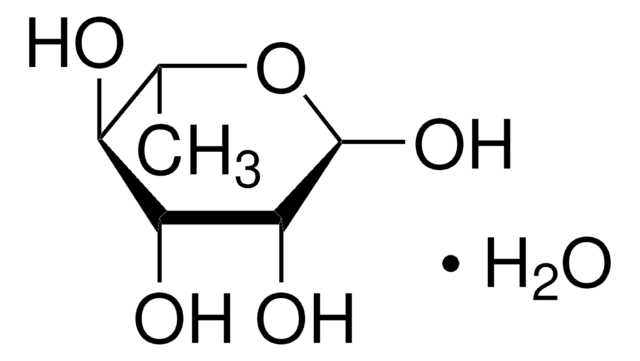
Mucic acid
97%
Synonym(s):
Galactaric acid, MTPA, Saccharolactic acid, Tetrahydroxyadipic acid, Tetrahydroxyhexanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOOC(CHOH)4COOH
CAS Number:
Molecular Weight:
210.14
Beilstein:
1728117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
powder
Assay:
97%
Recommended Products
Quality Level
Assay
97%
form
powder
mp
220-225 °C (dec.) (lit.)
SMILES string
O[C@@H]([C@@H](O)[C@H](O)C(O)=O)[C@@H](O)C(O)=O
InChI
1S/C6H10O8/c7-1(3(9)5(11)12)2(8)4(10)6(13)14/h1-4,7-10H,(H,11,12)(H,13,14)/t1-,2+,3+,4-
InChI key
DSLZVSRJTYRBFB-DUHBMQHGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Mucic acid or galactaric acid can be used as a precursor for the synthesis of:
It can be also utilized in the surface modification of monodisperse water-soluble magnetic nanoparticles.
- 2,3,4,5-tetra-O-acetylgalactaric acid (AGA) which is used as a monomer unit along with adipic acid for the synthesis of copolyanhydrides.
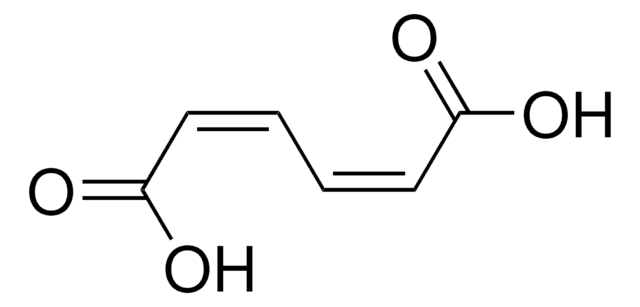
- Muconic acid and adipic acid.
It can be also utilized in the surface modification of monodisperse water-soluble magnetic nanoparticles.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Functionalization of monodisperse magnetic nanoparticles
Lattuada M and Hatton T A
Langmuir, 23(4), 2158-2168 (2007)
Highly Efficient Chemical Process To Convert Mucic Acid into Adipic Acid and DFT Studies of the Mechanism of the Rhenium-Catalyzed Deoxydehydration
Li X, et al.
Angewandte Chemie (International ed. in English), 53(16), 4200-4204 (2014)
B K Hubbard et al.
Biochemistry, 37(41), 14369-14375 (1998-10-17)
The genes encoding the enzymes in the (D)-glucarate/galactarate catabolic pathway have been identified in the Escherichia coli genome. These encode, in three transcriptional units, (D)-glucarate dehydratase (GlucD), galactarate dehydratase, 5-keto-4-deoxy-(D)-glucarate aldolase, tartronate semialdehyde reductase, a glycerate kinase that generates 2-phosphoglycerate
Andrea Lakatos et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(5), 1281-1290 (2004-03-10)
The Al(III)-binding abilities of two aldaric acids, D-saccharic acid and mucic acid (the neutral form is denoted as H(2)L), were studied in solution by means of pH potentiometric, (1)H and (13)C NMR, and ESI-MS techniques. The most probable conformations and
Harry Boer et al.
Applied microbiology and biotechnology, 86(3), 901-909 (2009-11-19)
There are at least three different pathways for the catabolism of D-galacturonate in microorganisms. In the oxidative pathway, which was described in some prokaryotic species, D-galacturonate is first oxidised to meso-galactarate (mucate) by a nicotinamide adenine dinucleotide (NAD)-dependent dehydrogenase (EC
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service