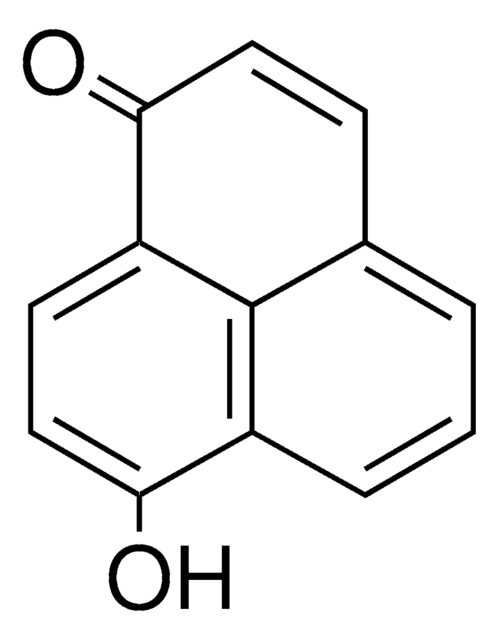
P10801
Perinaphthenone
97%
Synonym(s):
1H-Benzonaphthen-1-one, 7-Perinaphthenone, Phenalenone, Phenalone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H8O
CAS Number:
Molecular Weight:
180.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
153-156 °C (lit.)
SMILES string
O=C1C=Cc2cccc3cccc1c23
InChI
1S/C13H8O/c14-12-8-7-10-4-1-3-9-5-2-6-11(12)13(9)10/h1-8H
InChI key
WWBGWPHHLRSTFI-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francisco Arriagada et al.
Pharmaceutics, 12(4) (2020-04-25)
The incorporation of pigments and natural polyphenols into inorganic matrices, resulting in a hybrid material that improves the resistance and chemical stability of the pigments and the antioxidant capacity of the materials, has been of great interest to the pharmaceutical
S Opitz et al.
Planta, 216(5), 881-889 (2003-03-08)
Phenylphenalenones represent a typical group of secondary metabolites of the Haemodoraceae. Some of these phenolic compounds show organ-specific distribution within the plant. However, detailed information on cellular localisation is still lacking. To this end, confocal laser-scanning microscopy, microspectral photometry and
Mahmoud F Elsebai et al.
Natural product reports, 31(5), 628-645 (2014-04-02)
Covering up to the end of August 2013. Phenalenones are members of a unique class of natural polyketides exhibiting diverse biological potential. This is a comprehensive review of 72 phenalenones with diverse structural features originating from fungal sources. Their bioactive
Deski Beri et al.
Frontiers in chemistry, 8, 567-567 (2020-08-09)
Silicon nanocrystals (SiNCs) are regarded as a green and environmentally friendly material when compared with other semiconductor nanocrystals. Ultra-small SiNCs (with the size 4.6-5.2 nm) demonstrate strong UV absorption and photoluminescence in the near infrared (NIR) range with the high
Xiaopeng Chen et al.
Chemical communications (Cambridge, England), 47(9), 2628-2630 (2011-01-15)
Phenalenone derivatives were efficiently constructed from 1,8-diiodonaphthalene and tertiary propynols via a one-pot domino reaction which eventually included Pd-catalyzed Sonogoshira coupling, Pd-catalyzed allylic oxidation and Pd-catalyzed C(sp(2))-H activation. Moreover, the synthesized phenalenone derivative presented a practical application as a fluorescent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Cyclopenta[d,e,f]phenanthrene 97%](/deepweb/assets/sigmaaldrich/product/structures/107/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b.png)