114359
Bromocresol Green
ACS reagent, Dye content 95 %
Synonym(s):
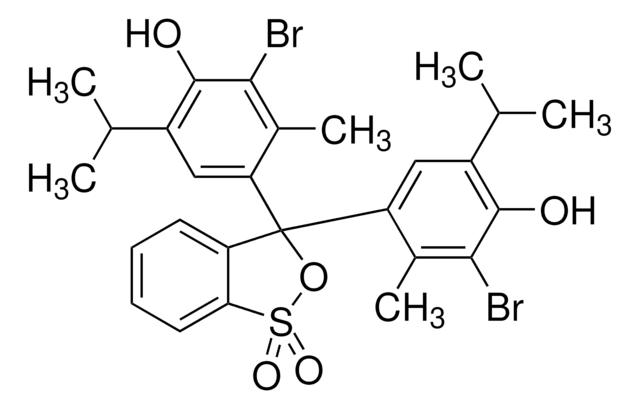
BCG; 2,6-dibromo-4-[3-(3,5-dibromo-4-hydroxy-2-methylphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-3-yl]-3-methylphenol
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Assay
≥95% (After Oxygen Combustion, silver nitrate titration)
form
powder
composition
Dye content, 95%
visual transition interval
3.8, yellow(passes test)
3.8-5.4, yellow to blue(passes test)
4.5, green
5.4, blue
mp
225 °C (dec.) (lit.)
λmax
423 nm
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Cc1c(Br)c(O)c(Br)cc1C2(OS(=O)(=O)c3ccccc23)c4cc(Br)c(O)c(Br)c4C
InChI
1S/C21H14Br4O5S/c1-9-12(7-14(22)19(26)17(9)24)21(13-8-15(23)20(27)18(25)10(13)2)11-5-3-4-6-16(11)31(28,29)30-21/h3-8,26-27H,1-2H3
InChI key
FRPHFZCDPYBUAU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- It is used widely as a vital stain to study blood-brain permeability.
- It has been used in the pH-sensitive colorimetric determination of glutamate decarboxylase activity.
- It has also been employed in the evaluation of paper-analytical devices for colorimetric analysis of serum samples.
- It is used as a tracking dye for DNA agarose gel electrophoresis, in protein determinations, and in charge-transfer complexation processes.
- It is used in TLC for the visualization of compounds with functional groups whose pKa is below 5.0.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service