95137
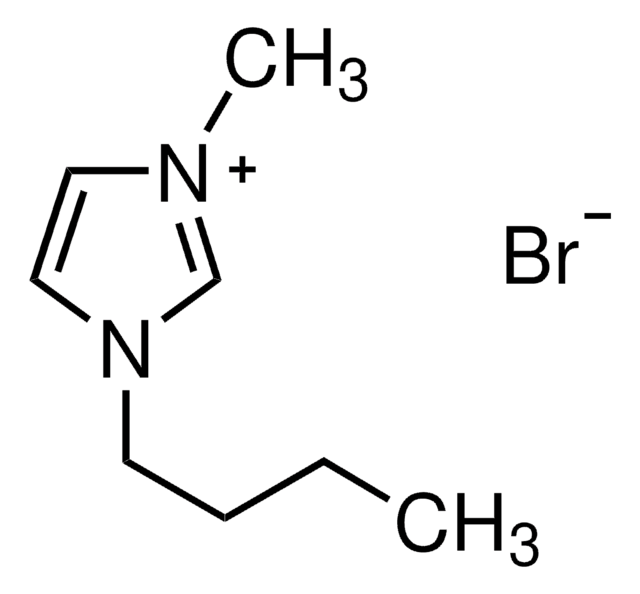
1-Butyl-3-methylimidazolium bromide
>97.0% (HPLC)
Synonym(s):
BMIMBr
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15BrN2
CAS Number:
Molecular Weight:
219.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
>97.0% (HPLC)
form
crystals
impurities
≤1% water
SMILES string
[Br-].CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.BrH/c1-3-4-5-10-7-6-9(2)8-10;/h6-8H,3-5H2,1-2H3;1H/q+1;/p-1
InChI key
KYCQOKLOSUBEJK-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
1-Butyl-3-methylimidazolium bromide is a neutral ionic liquid.
Application
BMIMBr undergoes gelation with gelatin to form ion jelly, a quasi-solid material for use in chemoresistive gas sensors. It can be used as a solvent for the preparation of 1,2,4,5-substituted imidazoles.
Other Notes
Ionic liquid used in a Heck reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Apparent molar volume and isentropic compressibility of ionic liquid 1-butyl-3-methylimidazolium bromide in water, methanol, and ethanol at T=(298.15 to 318.15) K
Zafarani-Moattar, Mohammed Taghi, and Hemayat Shekaari
The Journal of Chemical Thermodynamics, 37.10, 1029-1035 (2005)
Teng-Hui Wang et al.
International journal of molecular sciences, 22(2) (2021-01-23)
Mixtures of polyethylene oxide (PEO, M.W.~900,000) and imidazolium ionic liquids (ILs) are studied using high-pressure Fourier-transform infrared spectroscopy. At ambient pressure, the spectral features in the C-H stretching region reveal that PEO can disturb the local structures of the imidazolium
L. Xu et al. et al.
Organometallics, 19, 1123-1123 (2000)
Catalyst-free one-pot four component synthesis of polysubstituted imidazoles in neutral ionic liquid 1-butyl-3-methylimidazolium bromide.
Hasaninejad A, et al.
Journal of Combinatorial Chemistry, 12(6), 844-849 (2010)
Melting and freezing behaviors of prototype Ionic Liquids, 1-Butyl-3-methylimidazolium bromide and its chloride, studied by using a nano-Watt differential scanning calorimeter
Nishikawa, Keiko, et al.
The Journal of Physical Chemistry B, 111.18, 4894-4900 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service