C5732
CA-074
≥98% (HPLC)
Synonym(s):
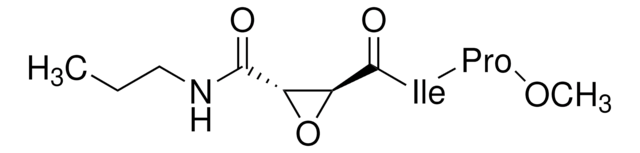
(L-3-trans-(Propylcarbamyl)oxirane-2-carbonyl)-L-isoleucyl-L-proline
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98% (HPLC)
form
powder
solubility
methanol: 1 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CCCNC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H]([C@H](C)CC)C(=O)N2CCC[C@H]2C(O)=O
InChI
1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10?,11-,12-,13-,14-/m0/s1
InChI key
ZEZGJKSEBRELAS-NSIINPIOSA-N
Application
- to study its effect on pseudotyped viruses bearing S proteins harboring R642M and N714K using HeLa cells
- to determine the role of autophagy in glucose deprivation (GD)-induced damage
- to study its effects on neuronal function upon exposure to His-cathepsin B (CATB)
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service