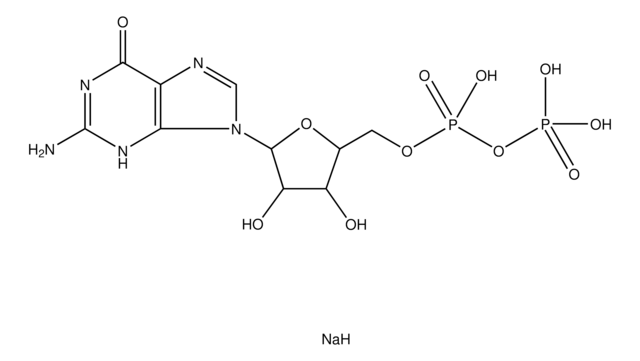
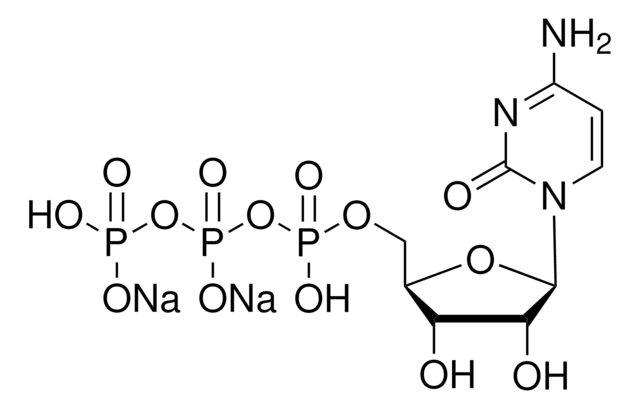
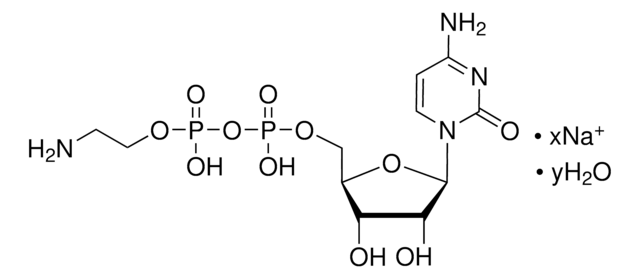
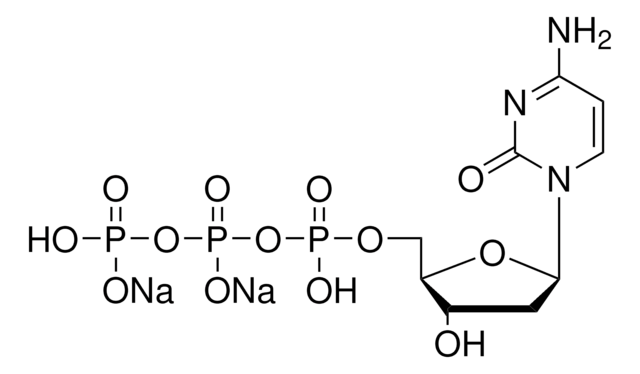
C9755
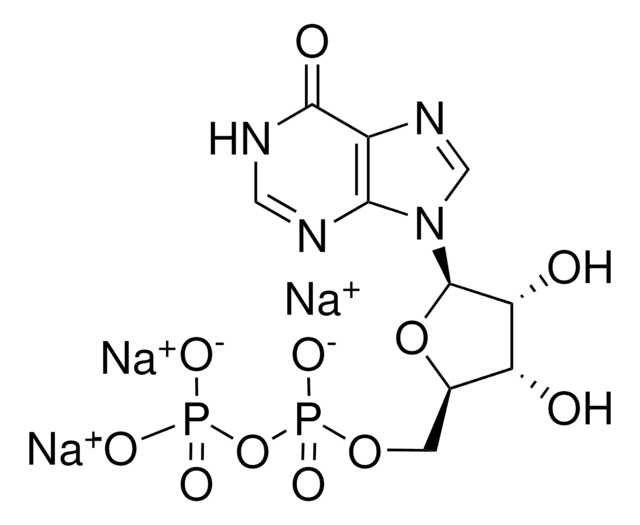
Cytidine 5′-diphosphate sodium salt hydrate
from yeast, ≥95%
Synonym(s):
CDP sodium salt hydrate
About This Item
Recommended Products
biological source
yeast
Quality Level
Assay
≥95%
form
powder
storage temp.
−20°C
SMILES string
[Na].NC1=NC(=O)N(C=C1)C2OC(COP(O)(=O)OP(O)(O)=O)C(O)C2O
InChI
1S/C9H15N3O11P2.Na.H/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(22-8)3-21-25(19,20)23-24(16,17)18;;/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H2,10,11,15)(H2,16,17,18);;
InChI key
MAGSIBHRUIBONY-UHFFFAOYSA-N
Related Categories
General description
Application
- as a substrate in nucleoside diphosphatase (NDPase) enzyme histochemistry assay of gold fish eye sections
- in ribonucleotide reductase assay
- dCDP formation assay.
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
ZIC®-cHILIC is a densely bonded zwitterionic stationary phase with phosphorylcholine functional groups covalently attached to silica.
HILIC separation is an alternative that permits sensitive MS detection and without the use of ion-pair reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service