Primary Human Hepatic Stellate Cells Culture Protocol
What are Hepatic Stellate Cells?
Hepatic Stellate Cells (HSCs), also called as vitamin A-storing cells, lipocytes, interstitial cells, fat-storing cells, or Ito cells, are liver cells that exist in the space between parenchymal cells and sinusoidal endothelial cells of the hepatic lobule. Stellate cells help store 80% of the vitamin A in the whole body as retinyl palmitate in lipid droplets in the cytoplasm. HSCs are also responsible for vitamin A homeostasis.
Primary Hepatic Stellate cells are isolated from whole human livers and are used by researchers to study liver cancer, fibrosis, and liver development and regeneration1. HSCs have a star-like appearance and upon activation can acquire contractile myofibroblast-like morphology, which is an essential step in fibrosis. In fibrosis, activated Stellate cells display a loss of stored vitamin A and increased collagen synthesis, along with other extracellular matrix components.
Our cryopreserved hepatic Stellate cells are derived from the human liver, with each donor providing documented consent for research use of non-transplantable organs or tissues. Cells are cryopreserved at the end of the primary culture. Each lot undergoes testing for specific cell markers and comes with a guarantee of ≥70% post-thaw viability. In this protocol, we demonstrate how to thaw, plate, and culture primary human hepatic Stellate cells.
Hepatic Stellate Culture Materials
- Normal Human Hepatic Stellate Cells
Note: Upon receipt, immediately store cryovial(s) in vapor phase liquid nitrogen. - Collagen Type I, Rat Tail
- Tissue culture treated multiwell plates
- 1X Stellate Medium2:
Hepatic Stellate Cells Growth Protocol
These protocols were performed using a Class II laminar flow biohood and an incubator set to 37°C and 5% CO2. Researchers should wear PPE such as safety glasses, gloves, and a lab coat.
Collagen Coated Plate Preparation
1. Dilute collagen to a final working concentration of 56µg/mL in sterile 70% ethanol and gently mix until the collagen is fully solubilized.
2. Add the collagen/ethanol mixture to each well to completely cover the bottom of wells.
3. Gently swirl the cell culture plate so the collagen/ethanol mixture evenly coats the wells.
4. Air dry plates in the laminar flow hood and leave over night with the cover slightly ajar to allow airflow and prevent condensation.
Thawing and Plating Hepatic Stellate Cells
For this protocol, handle cells gently and quickly to maintain viability. Collagen I coated cultureware is required for culturing Stellate cells (see above, Collagen Coated Plate Preparation)
1. Place vial in a 37˚C water bath and rotate the vial gently until the contents are thawed. Remove the vial from the water bath, wipe it dry, rinse with 70% ethanol, and transfer to the sterile work area. Being careful not to touch the interior threads with fingers, remove the cap.
2. Using a pipette, transfer contents to a sterile 15 ml conical tube.
3. Wash the vial with 5 ml of warmed stellate medium and add this to conical tube.
4. Centrifuge the conical at 250xg for 5 minutes. Then aspirate medium and re-suspend the pellet in fresh stellate medium.
5. Count cells using a cell counter such as the Scepter 3.0 Handheld Automated Cell Counter.
6. For expansion, seed the stellate cells at a density of 4,000 cells/cm2 on collagen I coated plates.
7. Do not disturb the culture for at least 12 hours after seeding for best results. Change the stellate growth medium the next day to remove any residual DMSO or unattached cells and every other day after.
Sub-Culturing Hepatic Stellate Cells Protocol
1. Subculture cells when they have reached 90% confluency. Cells can be monitored using the Millicel® DCI Digital Cell Imager.
2. Warm the 0.25% trypsin solution, Dulbecco’s Phosphate Buffered Saline, without Calcium & Magnesium (DPBS), and Stellate medium to room temperature.
3. Aspirate the medium and rinse the cells with DPBS. Add the trypsin solution into culture flask and incubate at 37˚C for 3-5 minutes or until the cells detach.
4. At the end of typsinization, wash cells off flask with the Stellate medium.
5. Transfer the cells to the centrifuge tube and centrifuge the tube at 250xg for 5 minutes.
6. After centrifugation, aspirate the medium, resuspend the pellet in 1-2 ml fresh medium, and count the cells for seeding.
7. Seed the Stellates cells at a density of 4,000 cells/cm2 on collagen I coated plates (see protocol above).
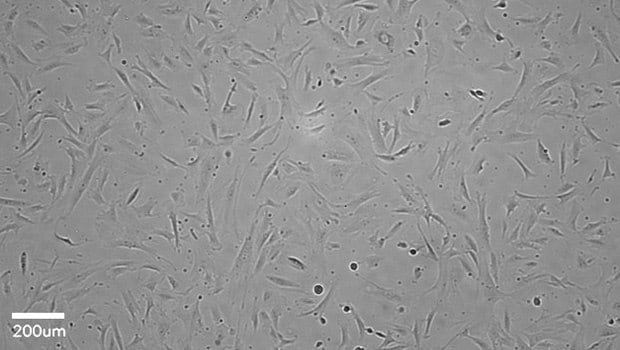
Figure 1.Stellate cells imaged after 4 days of incubation. Stellate cells were plated at a density of 4000 cells/cm2 on collagen-coated plate (08-115). Media was exchanged every other day and the cells were imaged with the Millicell® DCI Digital Cell Imager at 10x magnification setting. Stellate cells show a characteristic elongated shape.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?