104353
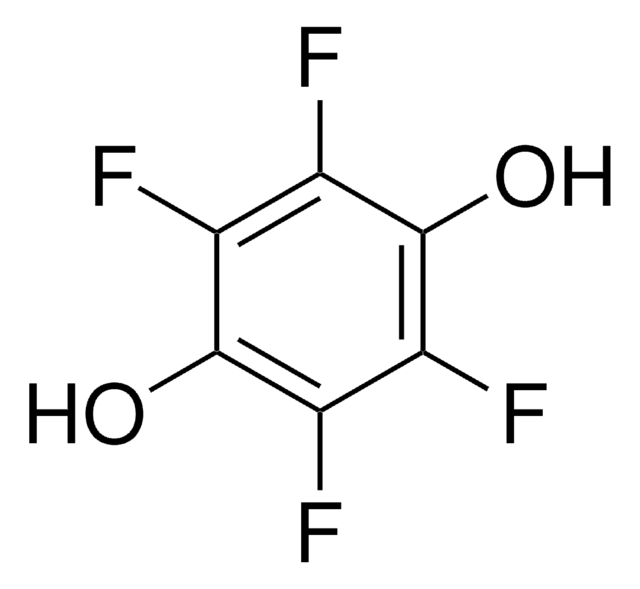
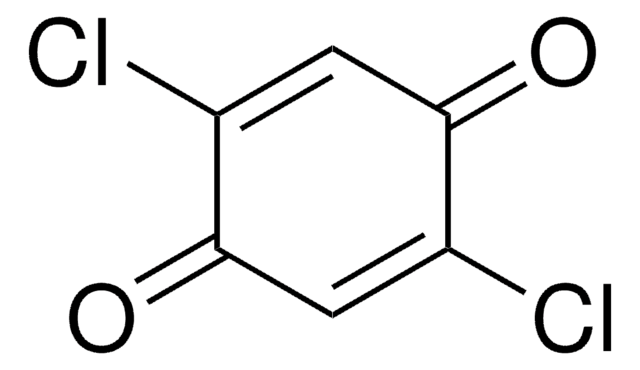
Tetrafluoro-1,4-benzoquinone
97%
Synonym(s):
2,3,5,6-Tetrafluoroquinone, Fluoranil, Tetrafluorobenzoquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6F4(=O)2
CAS Number:
Molecular Weight:
180.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
183-186 °C (subl.) (lit.)
functional group
fluoro
ketone
SMILES string
FC1=C(F)C(=O)C(F)=C(F)C1=O
InChI
1S/C6F4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI key
JKLYZOGJWVAIQS-UHFFFAOYSA-N
General description
Tetrafluoro-1,4-benzoquinone is a fluorinated building block, commonly used as a precursor for fluoro derivatives.
Application
Tetrafluoro-1,4-benzoquinone (fluoranil) can be used to prepare:
- Symmetrical or unsymmetrical ethers by coupling of two alcohols via the oxidation-reduction condensation reaction.
- Azocino[4,3-b]indole scaffold, which is used as an inetermediate to prepare (±)-dasycarpidone.
- Chiral and racemic charge-transfer (CT) complexes with binaphthol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Convenient Method for the Preparation of Symmetrical or Unsymmetrical Ethers by The Coupling of Two Alcohols via A New Type of Oxidation-reduction Condensation Using Tetrafluoro-1, 4-benzoquinone
Shintou T and Mukaiyama T
Chemistry Letters (Jpn), 32(11), 984-985 (2003)
A convenient method for the preparation of symmetrical or unsymmetrical ethers by the coupling of two alcohols via a new type of oxidation-reduction condensation using tetrafluoro-1, 4-benzoquinone.
Shintou T and Mukaiyama T.
Chemistry Letters (Jpn), 11, 984-985 (2003)
Ken Okamoto et al.
Journal of the American Chemical Society, 125(41), 12416-12417 (2003-10-09)
Self-promoted electron transfer from a cobalt(II) porphyrin [Co(II)OEP] to p-fluoranil (F4Q) occurs, exhibiting a second-order dependence of the electron-transfer rate with respect to the F4Q concentration due to the formation of a strong complex between the dimer radical anion [(F4Q)2*-]
Tetrafluoro-p-benzoquinone
Essers M and Haufe G
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Complexation Behavior of Binaphthol/Tetrafluoro-1, 4-benzoquinone Charge-Transfer Complex.
Imai Y, et al.
Crystal Growth & Design, 9(5), 2393-2397 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service