127574
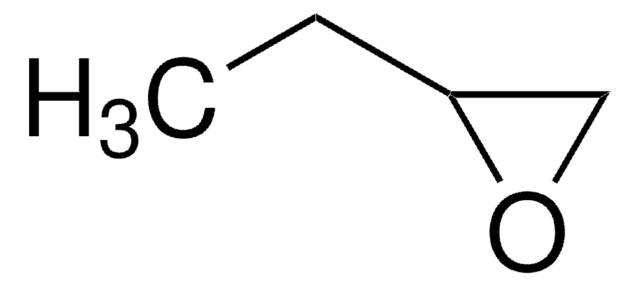
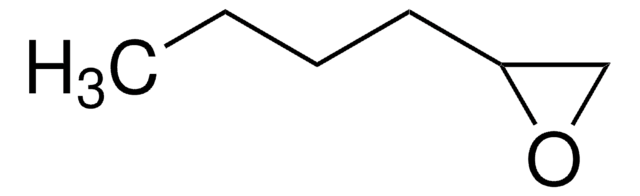
3,4-Epoxy-1-butene
98%
Synonym(s):
2-Vinyloxirane, 3,4-Epoxy-1-butene, Butadiene monoxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein:
103170
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
65-66 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC1CO1
InChI
1S/C4H6O/c1-2-4-3-5-4/h2,4H,1,3H2
InChI key
GXBYFVGCMPJVJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-58.0 °F - closed cup
Flash Point(C)
-50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Kowalczyk et al.
Chemical research in toxicology, 14(6), 746-753 (2001-06-21)
Hydroxyethyl adducts arising by the reactions of simple epoxides at the N1 position of adenine nucleosides can deaminate to give the inosine analogues which, if formed in DNA, are suspected of being highly mutagenic. A method has been developed for
Stereoselective ring expansion of vinyl oxiranes: mechanistic insights and natural product total synthesis.
Matthew Brichacek et al.
Angewandte Chemie (International ed. in English), 49(9), 1648-1651 (2010-02-06)
Eduardo Cemeli et al.
Mutation research, 664(1-2), 69-76 (2009-05-12)
The toxicity of butadiene and styrene is exerted by their metabolites. Such metabolites have been extensively scrutinized at the in vitro level demonstrating evident genotoxic properties. In monitoring, a diverse range of outcomes has been produced. Additionally, epidemiological studies in
M W Himmelstein et al.
Chemico-biological interactions, 135-136, 703-713 (2001-06-09)
(1-Chloroethenyl)oxirane (CEO) is a metabolite of beta-chloroprene (2-chloro-1,3-butadiene, CD). The purpose of this study was to evaluate the in vitro mutagenic and clastogenic (chromosome breaking) potential of CEO. For comparative purposes, the study also included an evaluation of the racemic
Thomas J L Mustard et al.
Journal of the American Chemical Society, 135(4), 1471-1475 (2013-01-01)
Density functional theory computations of the Cu-catalyzed ring expansion of vinyloxiranes is mediated by a traceless dual Cu(I)-catalyst mechanism. Overall, the reaction involves a monomeric Cu(I)-catalyst, but a single key step, the Cu migration, requires two Cu(I)-catalysts for the transformation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service