128759
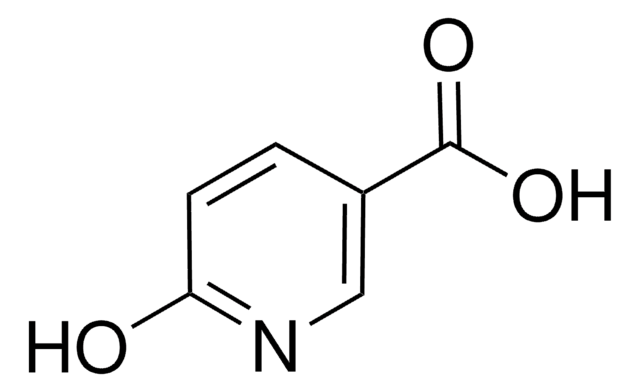
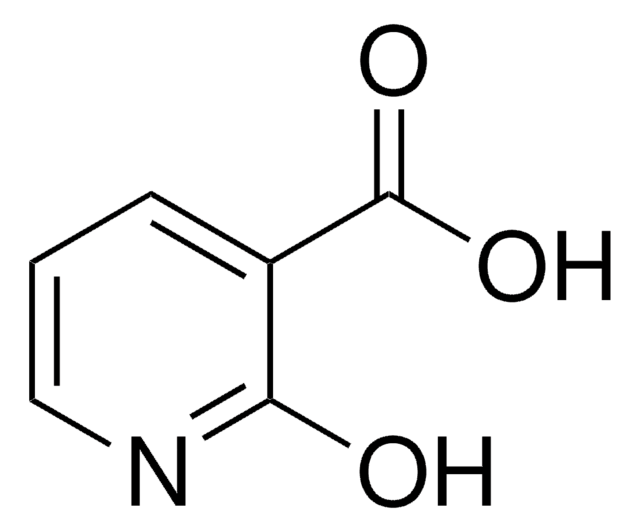
6-Hydroxypyridine-3-carboxylic acid
98%
Synonym(s):
2-Hydroxy-5-pyridinecarboxylic acid, 6-Hydroxynicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H5NO3
CAS Number:
Molecular Weight:
139.11
Beilstein:
115991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
>300 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc(O)nc1
InChI
1S/C6H5NO3/c8-5-2-1-4(3-7-5)6(9)10/h1-3H,(H,7,8)(H,9,10)
InChI key
BLHCMGRVFXRYRN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-Hydroxypyridine-3-carboxylic acid (6-Hydroxynicotinic acid) reacts with Ln(2)O(3) (Ln = Nd, Sm, Eu, Gd) and oxalic acid (H(2)OX) to generate four novel lanthanide-organic coordination polymeric networks.
Application
6-Hydroxypyridine-3-carboxylic acid (6-Hydroxynicotinic acid) was used in the synthesis of molecularly imprinted polymer (MIP).
Employed in the synthesis of retinoids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, 285-285 (1995)
Dayun Zhao et al.
Analytical and bioanalytical chemistry, 401(7), 2259-2273 (2011-08-27)
A new molecularly imprinted polymer (MIP) has been prepared on silica beads using the radical "grafting from" polymerization method for selective extraction of minor contaminant mycotoxin of patulin (PTL). After the introduction of amino groups onto the silica surface with
Cai-Ming Liu et al.
Dalton transactions (Cambridge, England : 2003), (29)(29), 5666-5672 (2010-05-08)
6-Hydroxypyridine-3-carboxylic acid (6-HOPy-3-CO(2)H) reacts with Ln(2)O(3) (Ln = Nd, Sm, Eu, Gd) and oxalic acid (H(2)OX) under hydrothermal conditions to generate four novel lanthanide-organic coordination polymeric networks [Ln(2)(1H-6-Opy-3-CO(2))(2)(OX)(2)(H(2)O)(3)] x 2.5 H(2)O (Ln = Nd, 1; Sm, 2; 1H-6-Opy-3-CO(2)(-) = 1-hydro-6-oxopyridine-3-carboxylate)
Li Xu et al.
Wei sheng wu xue bao = Acta microbiologica Sinica, 46(1), 63-67 (2006-04-04)
The current technology for microbial hydroxylation of nicotinic acid is a procedure of resting cells transformation. Here it was reported that non-degradation of 6-hydroxynicotinic acid during hydroxylation of nicotinic acid by resting cells was due to existing of nicotinic acid
Ashish Gupta et al.
NMR in biomedicine, 18(5), 293-299 (2005-03-11)
The utility of (1)H NMR spectroscopy is suggested and demonstrated for the diagnosis of Pseudomonas aeruginosa in urinary tract infection (UTI). The specific property of P. aeruginosa of metabolizing nicotinic acid to 6-hydroxynicotinic acid (6-OHNA) is exploited. The quantity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service