All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8
CAS Number:
Molecular Weight:
116.16
Beilstein:
635873
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
liquid
refractive index
n20/D 1.595 (lit.)
bp
181-182 °C (lit.)
mp
−5-−3 °C (lit.)
solubility
organic solvents: miscible
water: insoluble
density
0.996 g/mL at 25 °C (lit.)
storage temp.
2-8°C
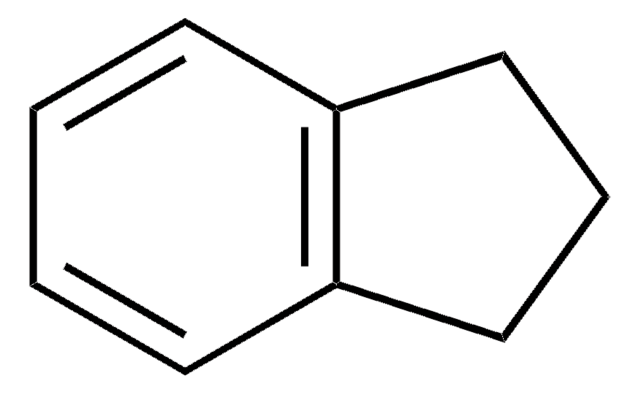
SMILES string
C1C=Cc2ccccc12
InChI
1S/C9H8/c1-2-5-9-7-3-6-8(9)4-1/h1-6H,7H2
InChI key
YBYIRNPNPLQARY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Indene is oxidized to mixtures of cis- and trans-indandiols and related metabolites by Pseudomonas putida and Rhodococcus sp.
Application
Indene was used in the synthesis of new C60 derivative, indene-C60 bisadduct. It was used in preparing polyindene by the controlled cationic polymerization initiated with cumyl methyl ether/TiCl4 in CH2Cl2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B C Buckland et al.
Metabolic engineering, 1(1), 63-74 (2000-08-10)
Indene is oxidized to mixtures of cis- and trans-indandiols and related metabolites by Pseudomonas putida and Rhodococcus sp. isolates. Indene metabolism is consistent with monooxygenase and dioxygenase activity. P. putida resolves enantiomeric mixtures of cis-1,2-indandiol by further selective oxidation of
High glass transition temperature polyolefins obtained by the catalytic hydrogenation of polyindene.
Hahn SF and Hillmyer MA.
Macromolecules, 36(1), 71-76 (2003)
David Schneider et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(50), 12243-12252 (2017-03-24)
A series of heteroleptic tris(cyclopentadienyl) Ce
Lucas J Gursky et al.
Applied microbiology and biotechnology, 85(4), 995-1004 (2009-07-02)
The styAB genes from Pseudomonas putida CA-3, which encode styrene monooxygenase, were subjected to three rounds of in vitro evolution using error-prone polymerase chain reaction with a view to improving the rate of styrene oxide and indene oxide formation. Improvements
Regioselective synthesis of indenols by rhodium-catalyzed C-H activation and carbocyclization of aryl ketones and alkynes.
Krishnamoorthy Muralirajan et al.
Angewandte Chemie (International ed. in English), 50(18), 4169-4172 (2011-03-31)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)