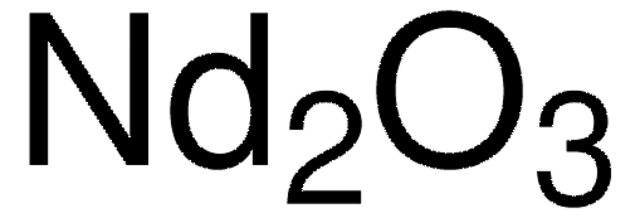203181
Dysprosium(III) oxide
≥99.99% trace metals basis
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
Dy2O3
CAS Number:
Molecular Weight:
373.00
EC Number:
MDL number:
UNSPSC Code:
12352303
eCl@ss:
38161201
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: dysprosium
density
7.81 g/mL at 25 °C (lit.)
SMILES string
O=[Dy]O[Dy]=O
InChI
1S/2Dy.3O
InChI key
NLQFUUYNQFMIJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Małgorzata Norek et al.
Journal of the American Chemical Society, 130(15), 5335-5340 (2008-03-22)
The transverse 1H relaxivities of aqueous colloidal solutions of dextran coated Dy2O3 nanoparticles of different sizes were investigated at magnetic field strengths (B) between 7 and 17.6 T. The particle size with the maximum relaxivity (r2) appears to vary between
Masoud Salavati-Niasari et al.
Ultrasonics sonochemistry, 17(5), 870-877 (2010-03-23)
Dysprosium carbonates nanoparticles were synthesized by the reaction of dysprosium acetate and NaHCO(3) by a sonochemical method. Dysprosium oxide nanoparticles with average size about 17 nm were prepared from calcination of Dy(2)(CO(3))(3).1.7H(2)O nanoparticles. Dy(OH)(3) nanotubes were synthesized by sonication of
S E Seltzer et al.
Journal of computer assisted tomography, 5(3), 370-374 (1981-06-01)
We used a stepwise approach to identify, design, synthesize, and test new high atomic number particulate contrast agents that would be especially well suited for use with computed tomography (CT). Our goal was to produce extremely radiopaque compounds with highly
Happy et al.
Journal of nanoscience and nanotechnology, 7(3), 907-915 (2007-04-25)
The experimental parameters that control the size and size distribution of dysprosium oxide nanoparticles synthesized by homogeneous precipitation technique have been systematically investigated. The particles were characterized with respect to their size, shape, and thermal decomposition behavior. It was found
J M Peeters et al.
Physics in medicine and biology, 51(6), N127-N137 (2006-03-03)
Susceptibility markers for passive tracking need to be small in order to maintain the shape and mechanical properties of the endovascular device. Nevertheless, they also must have a high magnetic moment to induce an adequate artefact at a variety of
Articles
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service