All Photos(1)
About This Item
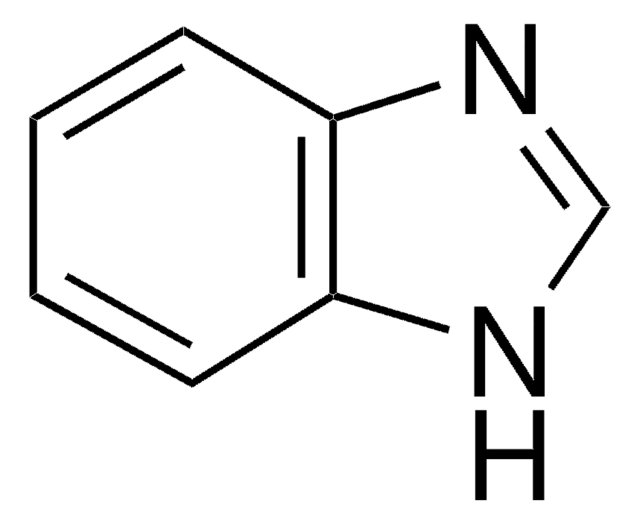
Empirical Formula (Hill Notation):
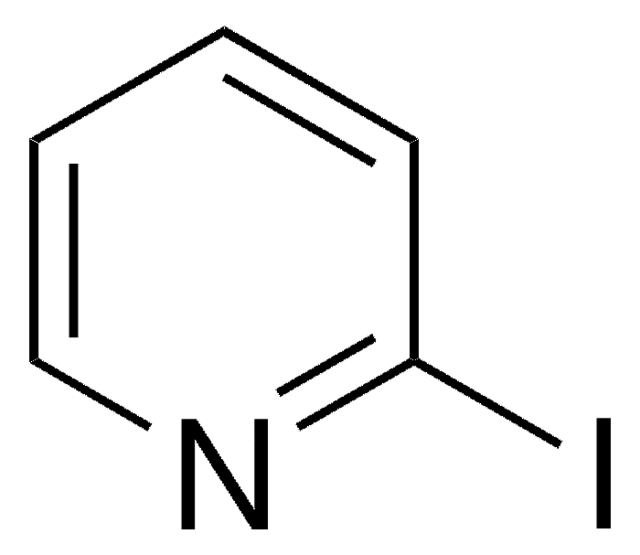
C7H6N2
CAS Number:
Molecular Weight:
118.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.626 (lit.)
bp
103 °C/1 mmHg (lit.)
density
1.165 g/mL at 25 °C (lit.)
SMILES string
c1ccn2ccnc2c1
InChI
1S/C7H6N2/c1-2-5-9-6-4-8-7(9)3-1/h1-6H
InChI key
UTCSSFWDNNEEBH-UHFFFAOYSA-N
Related Categories
General description
In vivo anti-trypanosomal activity of imidazo[1,2-a]pyridiness in the STIB900 mouse model has been investigated.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Martina Hieke et al.
Bioorganic & medicinal chemistry letters, 22(5), 1969-1975 (2012-02-14)
A novel class of 5-lipoxygenase (5-LO) inhibitors characterized by a central imidazo[1,2-a]pyridine scaffold, a cyclohexyl moiety and an aromatic system, is presented. This scaffold was identified in a virtual screening study and exhibits promising inhibitory potential on the 5-LO. Here
Sophie Marhadour et al.
European journal of medicinal chemistry, 58, 543-556 (2012-11-21)
A novel series of 2,3-diarylimidazo[1,2-a]pyridines was synthesized and evaluated for their antileishmanial activities. Four derivatives exhibited good activity against the promastigote and intracellular amastigote stages of Leishmania major, coupled with a low cytotoxicity against the HeLa human cell line. The
Enza Palazzo et al.
Neuropharmacology, 58(3), 660-667 (2009-12-01)
The 6-methoxy-2-phenylimidazo[1,2-b]pyridazine-3-carboxylic acid, DM2, exerts anti-absence activity and blocks Cav3.1 channel, a T-type voltage-dependent Ca(2+) channel subtype, in vitro. The current study investigated the effect of intra-ventrolateral periaqueductal grey (VLPAG) administration of DM2 on formalin-induced nocifensive responses in rats. In
Joanna M Wisniewska et al.
Biochemical pharmacology, 83(2), 228-240 (2011-10-27)
5-Lipoxygenase (5-LO) is a crucial enzyme of the arachidonic acid (AA) cascade and catalyzes the formation of bioactive leukotrienes (LTs) which are involved in inflammatory diseases and allergic reactions. The pathophysiological effects of LTs are considered to be prevented by
Yoshito Terao et al.
Bioorganic & medicinal chemistry letters, 22(24), 7326-7329 (2012-11-14)
Imidazo[1,2-a]pyridine derivatives were designed, synthesized, and evaluated as inhibitors of the apoptosis signal-regulating kinase 1 (ASK1). These were based on a benzothiazole derivative that was discovered from high-throughput screening of our compound library. As a result, we identified potent, selective
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)
![Imidazo[1,2-a]pyrazine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/804/1712d71f-52fb-4758-9a22-85b6c96cd4e8/640/1712d71f-52fb-4758-9a22-85b6c96cd4e8.png)
![Imidazo[1,2-a]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/187/001/4862c14e-bec7-4475-85a5-f178e48ff60f/640/4862c14e-bec7-4475-85a5-f178e48ff60f.png)