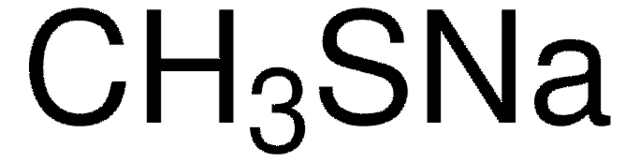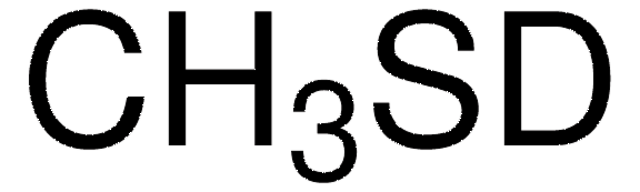281018
Sodium thiomethoxide
95%
Synonym(s):
Sodium methanethiolate, Methanethiol sodium salt, Sodium thiomethoxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3SNa
CAS Number:
Molecular Weight:
70.09
Beilstein:
3592983
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
95%
form
powder or crystals
reaction suitability
core: sodium
SMILES string
[Na+].C[S-]
InChI
1S/CH4S.Na/c1-2;/h2H,1H3;/q;+1/p-1
InChI key
RMBAVIFYHOYIFM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sodium thiomethoxide is mainly used as a reagent in organicsynthesis, especially as a strong nucleophile for various reactions such astransesterification reactions and condensation reactions. It is also asulfur source to prepare various inorganic and organosulfurcompounds.
Application
Sodium thiomethoxide can be used:
- As a precursor to synthesize sulfonyl-containing dipolar glass polymers.
- To fabricate semiconductors for organic field effect transistors.
- To synthesize mono- and dithiols of tetraethylene glycol andpoly(ethylene glycol)s via transesterification reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Sol. 1 - Skin Corr. 1A
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dialkylated dibenzotetrathienoacene derivative as semiconductor for organic field effect transistors
Xiaoxia Liu, et al.
Organic Electronics, 15, 156-161 (2014)
High Dielectric Constant Sulfonyl-Containing Dipolar Glass Polymers with Enhanced Orientational Polarization
Zhu, Yu-Feng, et al.
Macromolecules, 51, 6257-6266 (2018)
Kuhan Chandru et al.
Scientific reports, 6, 29883-29883 (2016-07-23)
Thioesters and thioacetic acid (TAA) have been invoked as key reagents for the origin of life as activated forms of acetate analogous to acetyl-CoA. These species could have served as high-energy group-transfer reagents and allowed carbon insertions to form higher
Synthetic Communications, 37, 409-409 (2007)
Synthesis of Mono-and Dithiols of Tetraethylene Glycol and Poly(ethylene glycol)s via Enzyme Catalysis
Prajakatta Mulay, et al.
Catalysts, 9, 228-228 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service