330469
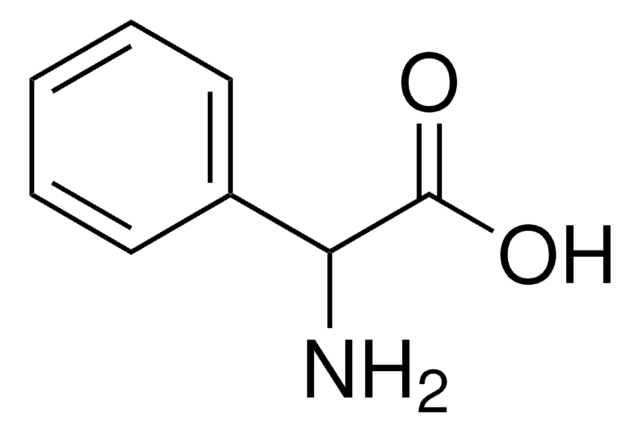
N-Phenylglycine
97%
Synonym(s):
(Phenylamino)acetic acid, Anilinoacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5NHCH2COOH
CAS Number:
Molecular Weight:
151.16
Beilstein:
509838
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: solution phase peptide synthesis
mp
121-123 °C (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNc1ccccc1
InChI
1S/C8H9NO2/c10-8(11)6-9-7-4-2-1-3-5-7/h1-5,9H,6H2,(H,10,11)
InChI key
NPKSPKHJBVJUKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G E Schumacher et al.
Journal of dental research, 76(1), 602-609 (1997-01-01)
Effective composite-to-dentin bonding has been achieved by the sequential use of dilute aqueous nitric acid (HNO3) and acetone solutions of N-phenylglycine and a carboxylic acid monomer, e.g., p-PMDM. Both the HNO3 pre-treatment and the surface-initiated polymerization that results from reaction
R E Webb et al.
Journal of dental research, 70(3), 211-214 (1991-03-01)
Three structurally related substituted amino acids (N-compounds) were studied in a three-step dentin-bonding protocol. The first step of an acidic ferric oxalate solution and the third step of a surface-active comonomer were held constant throughout the study. In the second
J M Janusz et al.
Journal of medicinal chemistry, 33(3), 1052-1061 (1990-03-01)
Twenty esters of L-aspartyl-D-phenylglycine, as well as two substituted analogues, an o-fluoro and a p-hydroxy-phenylglycine ester, were prepared. The L-aspartyl-D-phenylglycine (-)-alpha- and (+)-beta-fenchyl esters had the highest sweetness potency at 1200 and 3700 times that of sucrose, respectively. The high
Synthesis of cyclic and acyclic beta-amino acids via chelation-controlled 1,3-dipolar cycloaddition.
Roger Hanselmann et al.
The Journal of organic chemistry, 68(22), 8739-8741 (2003-10-25)
Isoxazolidines have been synthesized in diastereomeric excess up to 94% via a MgBr2-induced chelation-controlled 1,3-dipolar cycloaddition reaction with N-hydroxyphenylglycinol as a chiral auxiliary. The diastereomerically pure isoxazolidines were further transformed into cyclic and acyclic beta-amino acid derivatives.
J Watkins et al.
Trends in pharmacological sciences, 15(9), 333-342 (1994-09-01)
Metabotropic glutamate receptors represent a family of G protein-coupled receptors that can be activated by L-glutamate, the principal excitatory neurotransmitter in the brain. Until recently, progress in identifying the physiological and pathological roles of metabotropic glutamate receptors has been hampered
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service