345849
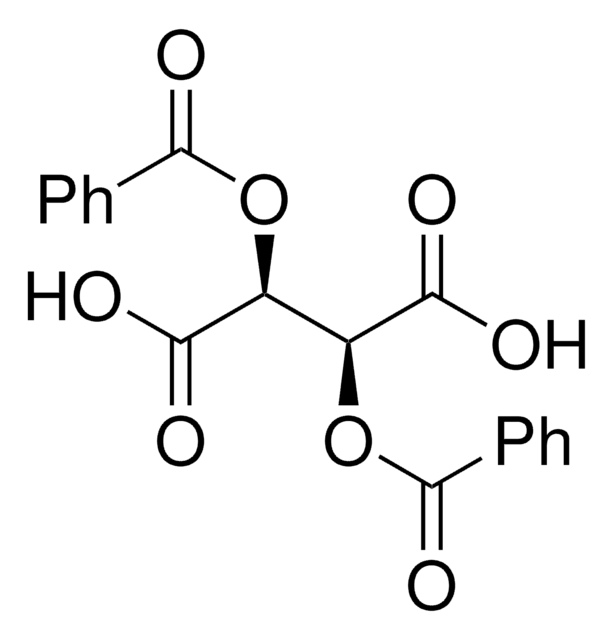
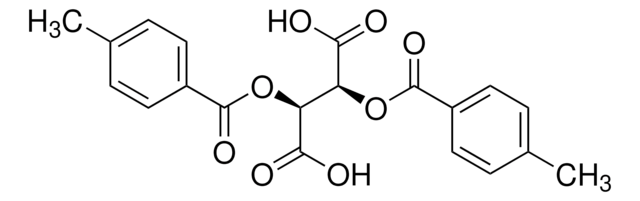
Dibenzoyl-L-tartaric acid
98%
Synonym(s):
L-DBTA, (−)-O,O′-Dibenzoyl-L-tartaric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
[C6H5CO2CH(CO2H)-]2
CAS Number:
Molecular Weight:
358.30
Beilstein:
709854
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
optical activity
[α]20/D −116°, c = 9 in ethanol
mp
152-155 °C (lit.)
functional group
carboxylic acid
ester
phenyl
SMILES string
OC(=O)[C@H](OC(=O)c1ccccc1)[C@@H](OC(=O)c2ccccc2)C(O)=O
InChI
1S/C18H14O8/c19-15(20)13(25-17(23)11-7-3-1-4-8-11)14(16(21)22)26-18(24)12-9-5-2-6-10-12/h1-10,13-14H,(H,19,20)(H,21,22)/t13-,14-/m1/s1
InChI key
YONLFQNRGZXBBF-ZIAGYGMSSA-N
Application
Dibenzoyl-L-tartaric acid may be used as a chiral resolving agent for the resolution of racemic Troger base. It may also be used as a ligand to synthesize chiral transition metal complexes, which have potential utility in organic asymmetric catalysis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Transition metal complexes of dibenzoyl-L-tartaric acid (db-L-tarH2) and L-tartaric acid (L-tarH2); X-ray crystal structure of {[Cu(L-tar)(phen)]?6H2O}n (phen=1,10-phenanthroline).
McCann M, et al.
Polyhedron, 16(20), 3655-3661 (1997)
A convenient method for the synthesis and resolution of Troger base.
Satishkumar S and Periasamy M.
Tetrahedron Asymmetry, 17(7), 1116-1119 (2006)
Stanley I Goldberg
Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life, 43(1), 31-37 (2013-01-25)
Construction and operation of a laboratory model, which combines the lately discovered enantioenrichment method of the author (2007) with the sun-powered evaporative pumping process of Hsu and Siegenthaler (Sedimentology 12:11-25 1969), is described. The model operated continuously for 120 days
C Q Cao et al.
European journal of pharmacology, 418(1-2), 79-87 (2001-05-04)
Due to low central nervous system (CNS) bioavailability of delta-opioid peptides, little is known about the effect of systemic administration of delta-opioid receptor ligands. The present study examined the effect of non-peptidergic delta-opioid receptor agonists, (+)-4-[(alphaR)-alpha-((2R,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80) and (-)dibenzoyl-L-tartaric acid
Emily M Jutkiewicz et al.
The Journal of pharmacology and experimental therapeutics, 309(1), 173-181 (2004-01-15)
The diarylpiperazine delta-opioid agonist SNC80 [(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide] produces convulsions, antidepressant-like effects, and locomotor stimulation in rats. The present study compared the behavioral effects in Sprague-Dawley rats of SNC80 with its two derivatives, SNC86 [(+)-4-[alpha(R)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide] and SNC162 [(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-phenyl)methyl]-N,N-diethylbenzamide], which differ by one
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service