All Photos(2)
About This Item
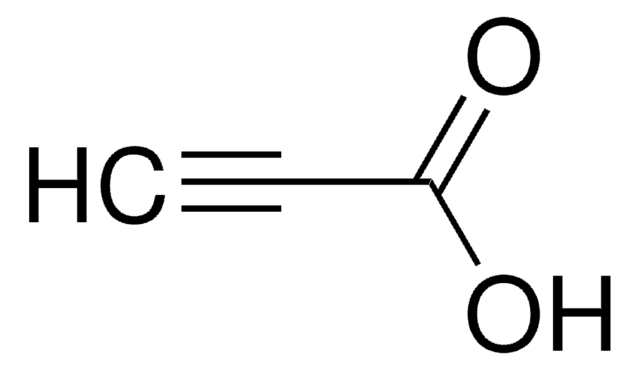
Linear Formula:
HC≡CCH2CH(COOCH3)2
CAS Number:
Molecular Weight:
170.16
Beilstein:
3539408
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (GC)
form
liquid
refractive index
n20/D 1.444
bp
93-95 °C/7 mmHg (lit.)
density
1.119 g/mL at 20 °C (lit.)
functional group
ester
SMILES string
COC(=O)C(CC#C)C(=O)OC
InChI
1S/C8H10O4/c1-4-5-6(7(9)11-2)8(10)12-3/h1,6H,5H2,2-3H3
InChI key
PWQAXFWWMXTVFT-UHFFFAOYSA-N
Application
Dimethyl propargylmalonate can be used as a reactant to synthesize:
- Nitro methylenecyclopentanes by [3+2] annulation reaction with various nitroalkenes in the presence of Triton B.
- Propargylmalonamides intermediates, applicable in the preparation of ″click BOX″ ligands by copper-catalyzed cycloaddition and oxazoline ring formation reaction.
- Cyclopentene derivatives by reacting with various α, β-unsaturated ketones using a combination of organocatalysts and transition metal catalysts.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of ?Click BOX? ligands and preliminary results on their application in the asymmetric copper catalyzed Henry reaction of o-methoxybenzaldehyde
Giunta D, et al.
Results in Chemistry, 3, 100122-100122 (2021)
Combination iminium, enamine and copper (I) cascade catalysis: a carboannulation for the synthesis of cyclopentenes
Yang T, et al.
Chemical Communications (Cambridge, England), (25), 2923-2925 (2008)
One-pot Michael addition/intramolecular carbocyclization of dimethyl propargylmalonate with nitroalkenes: A new stereoselective [3+ 2] annulation to 1-nitro 2-methylenecyclopentanes
Guillaume M, et al.
Synlett, 2002(11), 1883-1885 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service