T30007
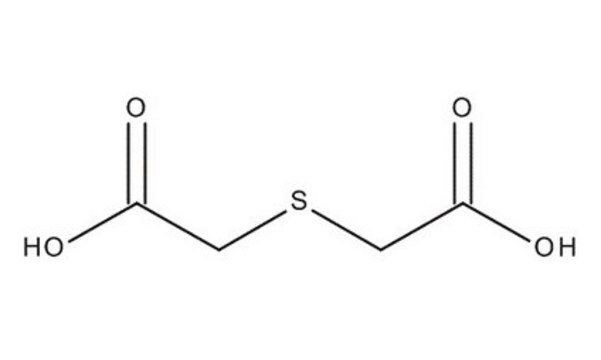
2,2′-Thiodiacetic acid
98%
Synonym(s):
2,2′-Thio-bis(acetic acid), Dicarboxydimethyl sulfide, Thiodiglycolic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
S(CH2COOH)2
CAS Number:
Molecular Weight:
150.15
Beilstein:
1764392
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
128-131 °C (lit.)
SMILES string
OC(=O)CSCC(O)=O
InChI
1S/C4H6O4S/c5-3(6)1-9-2-4(7)8/h1-2H2,(H,5,6)(H,7,8)
InChI key
UVZICZIVKIMRNE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
<ul>
<li><strong>Environmentally Safe Herbicides:</strong> It is used in the synthesis of quaternary ammonium mono- and bis-salts. This compound has been formulated into ammonium 2,2-thiodiacetates, serving as selective and environmentally safe herbicides. Its application underscores its utility in sustainable agriculture and safety in environmental management (Balczewski et al., 2018).</li>
</ul>
<li><strong>Environmentally Safe Herbicides:</strong> It is used in the synthesis of quaternary ammonium mono- and bis-salts. This compound has been formulated into ammonium 2,2-thiodiacetates, serving as selective and environmentally safe herbicides. Its application underscores its utility in sustainable agriculture and safety in environmental management (Balczewski et al., 2018).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thiodiglycolic acid as a possible causative agent of fixed drug eruption provoked only after continuous administration of S-carboxymethyl-L-cysteine: case report and review of reported cases.
A Adachi et al.
The British journal of dermatology, 153(1), 226-228 (2005-07-21)
T Lee et al.
Biotechnology progress, 16(3), 363-367 (2000-06-03)
A Gram-negative bacterium, Alcaligenes xylosoxydans ssp. xylosoxydans (SH91), consumed thiodiglycol (TDG), the nontoxic hydrolysis product of sulfur mustard, as a primary carbon source and transformed TDG to commercially relevant chemical precursors, [(2-hydroxyethyl)thio]acetic acid (HETA) and thiodiglycolic acid (TDGA). Aerobic fed
L W Wormhoudt et al.
Drug metabolism and disposition: the biological fate of chemicals, 25(4), 508-515 (1997-04-01)
1,2-Dibromoethane (1,2-DBE) is a carcinogenic compound that is metabolized both by cytochrome P450 (P450) and glutathione S-transferase (GST) enzymes, and that has been used by us as a model compound to study interindividual variability in biotransformation reactions. In this study
T M Visarius et al.
Drug metabolism and disposition: the biological fate of chemicals, 26(3), 193-196 (1998-04-04)
Thiodiglycolic acid has been identified as a major metabolite of the anticancer drug ifosfamide in humans. Patients treated with 12-16 g ifosfamide/m2.day excreted thiodiglycolic acid ranging from 0.10 +/- 0.02 mmol on the first day of therapy, to a maximum
Isotachophoresis.
L Krivánková et al.
Methods in enzymology, 270, 375-401 (1996-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service