All Photos(1)
About This Item
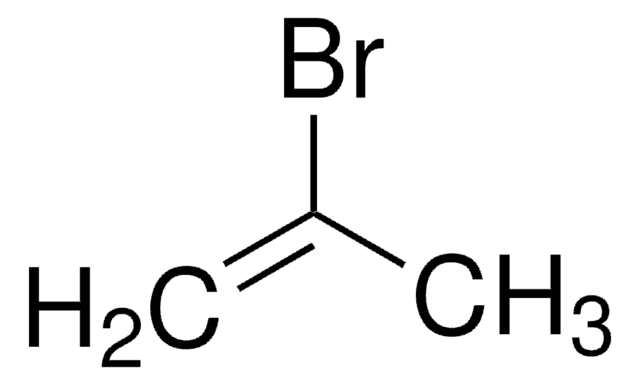
Linear Formula:
CH2=CHBr
CAS Number:
Molecular Weight:
106.95
Beilstein:
1361370
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
3.8 (15 °C, vs air)
Quality Level
vapor pressure
1551 mmHg ( 37.8 °C)
Assay
98%
autoignition temp.
986 °F
contains
200 ppm monomethyl ether hydroquinone as inhibitor
expl. lim.
15 %
bp
16 °C/750 mmHg (lit.)
mp
−139 °C (lit.)
density
1.517 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChI key
INLLPKCGLOXCIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- FTIR and Raman Spectroscopy Study of Soot Deposits: Investigates soot formation during the infrared multiphoton dissociation of vinyl bromide, suggesting potential applications in materials science regarding soot characteristics (Samoudi et al., 2022).
- Nickel-Catalyzed Reductive Cross-Coupling: Describes a method for coupling vinyl bromides with unactivated alkyl halides, highlighting its utility in synthetic organic chemistry for creating complex molecules (Gong et al., 2017).
- Silver-Promoted Synthesis of Vinyl Sulfones: This study explores the reactivity of vinyl bromides with sulfonyl hydrazides under aqueous conditions, applicable to pharmaceutical synthesis due to the biorelevance of sulfones (Zhang et al., 2020).
- Visible Light on Vinyl Halides: Examines the photocatalytic properties of vinyl bromides, which could influence the development of green chemistry applications (Pagire et al., 2020).
- Palladium Catalyzed Cross-Coupling of Diboronates: Focuses on the reactions of vinyl bromides with diboronates, offering insights into new methodologies for constructing biologically active compounds (Li et al., 2014).
Other Notes
V1902-100 g, V1902-900 g, and 1902-2.2 kg includes installed 316SS needle valve.
Recommended products
Stainless steel hose adapter Z146838 or stainless steel body mini gas regulator Z513547 is recommended.
hose barb
Product No.
Description
Pricing
regulator
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 3
Flash Point(F)
55.4 °F
Flash Point(C)
13 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
James A Marshall et al.
Organic letters, 6(3), 445-448 (2004-01-30)
[reaction: see text] Additions of vinylic zinc bromide reagents to alpha-chiral aldehydes (R(1) = CH(2)OTBS, R(2) = Me; R(1) = Me, R(2) = OTBS) in the presence of lithiated (+)- or (-)-N-methylephedrine proceed with predominant reagent control to afford anti
Mina Lee et al.
The Journal of chemical physics, 123(17), 174310-174310 (2005-12-27)
The vibrational spectrum of the vinyl bromide cation in the first excited electronic state A 2A' was obtained by one-photon mass-analyzed threshold ionization (MATI) spectroscopy. The use of an improved vacuum-ultraviolet radiation source based on four-wave sum frequency mixing in
Bryden A F Le Bailly et al.
Chemical communications (Cambridge, England), 48(10), 1580-1582 (2011-11-02)
An iron-catalysed, hydride-mediated reductive cross-coupling reaction has been developed for the preparation of alkanes. Using a bench-stable iron(II) pre-catalyst, reductive cross-coupling of vinyl iodides, bromides and chlorides with aryl- and alkyl Grignard reagents successfully gave the products of formal sp(3)-sp(3)
Qiwu Zhao et al.
The Journal of organic chemistry, 74(1), 459-462 (2008-11-27)
With CuI as the catalyst and K(3)PO(4) x 3 H(2)O as the base, highly efficient intramolecular S-vinylation of thiols with vinyl chlorides or bromides was successfully implemented without the help of an additional ligand. Moreover, the competition experiments revealed that
Changhui Sun et al.
Organic letters, 11(18), 4084-4087 (2009-08-20)
With the catalysis of CuI/trans-N,N'-dimethylcyclohexane-1,2-diamine, a number of carboxylic acids underwent efficient intramolecular O-vinylation with vinyl bromides leading to the synthesis of the corresponding five- and six-membered enol lactones. The same catalytic system also led to the efficient cycloisomerization of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service